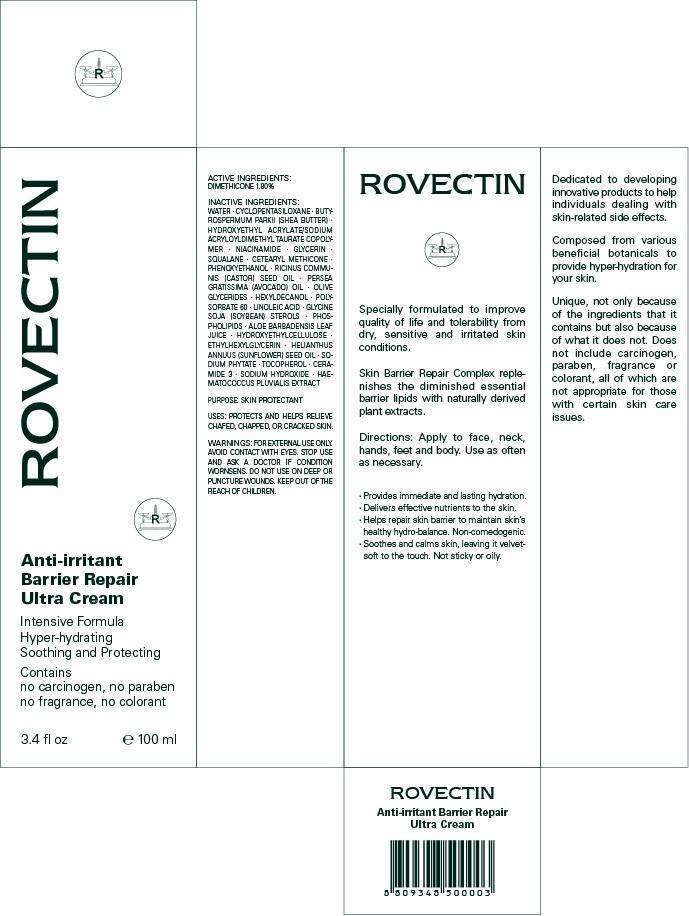
ROVECTIN
SECOND FORMULA INC.
SECOND FORMULA INC.
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- ROVECTIN INDICATIONS AND USAGE
- ROVECTIN DOSAGE AND ADMINISTRATION
- PACKAGE LABEL. PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT
ACTIVE INGREDIENT: DIMETHICONE 1.80%
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:
WATER, CYCLOPENTASILOXANE, BUTYROSPERMUM PARKII (SHEA BUTTER), HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, NIACINAMIDE, GLYCERIN, SQUALANE, CETEARYL METHICONE, PHENOXYETHANOL, RICINUS COMMUNIS (CASTOR) SEED OIL, PERSEA GRATISSIMA (AVOCADO) OIL, OLIVE GLYCERIDES, HEXYLDECANOL, POLYSORBATE 60, LINOLEIC ACID, GLYCINE SOJA (SOYBEAN) STEROLS, PHOSPHOLIPIDS, ALOE BARBADENSIS LEAF JUICE, HYDROXYETHYLCELLULOSE, ETHYLHEXYLGLYCERIN, HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL, SODIUM PHYTATE, TOCOPHEROL, CERAMIDE 3, SODIUM HYDROXIDE, HAEMATOCOCCUS PLUVIALIS EXTRACT
PURPOSE
PURPOSE: SKIN PROTECTANT
WARNINGS
WARNINGS:
FOR EXTERNAL USE ONLY.
AVOID CONTACT WITH EYES.
STOP USE AND ASK A DOCTOR IF CONDITION WORNSENS.
DO NOT USE ON DEEP OR PUNCTURE WOUNDS.
KEEP OUT OF THE REACH OF CHILDREN.
KEEP OUT OF REACH OF CHILDREN
KEEP OUT OF REACH OF CHILDREN
ROVECTIN INDICATIONS AND USAGE
Directions:
Apply to face, neck, hands, feet and body.
Use as often as necessary.
ROVECTIN DOSAGE AND ADMINISTRATION
Directions:
Apply to face, neck, hands, feet and body.
Use as often as necessary.
PACKAGE LABEL. PRINCIPAL DISPLAY PANEL
ROVECTINDimethicone CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||