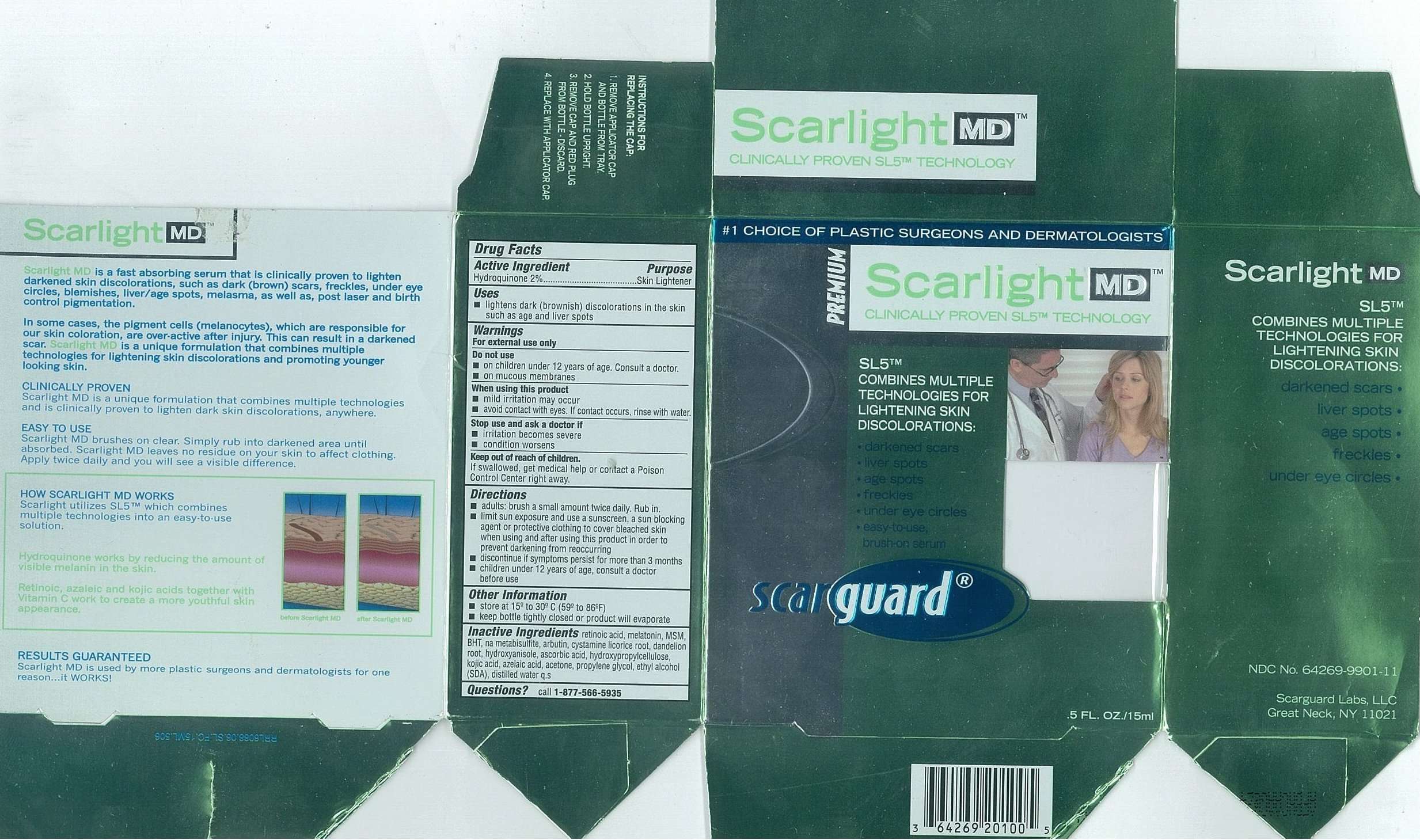
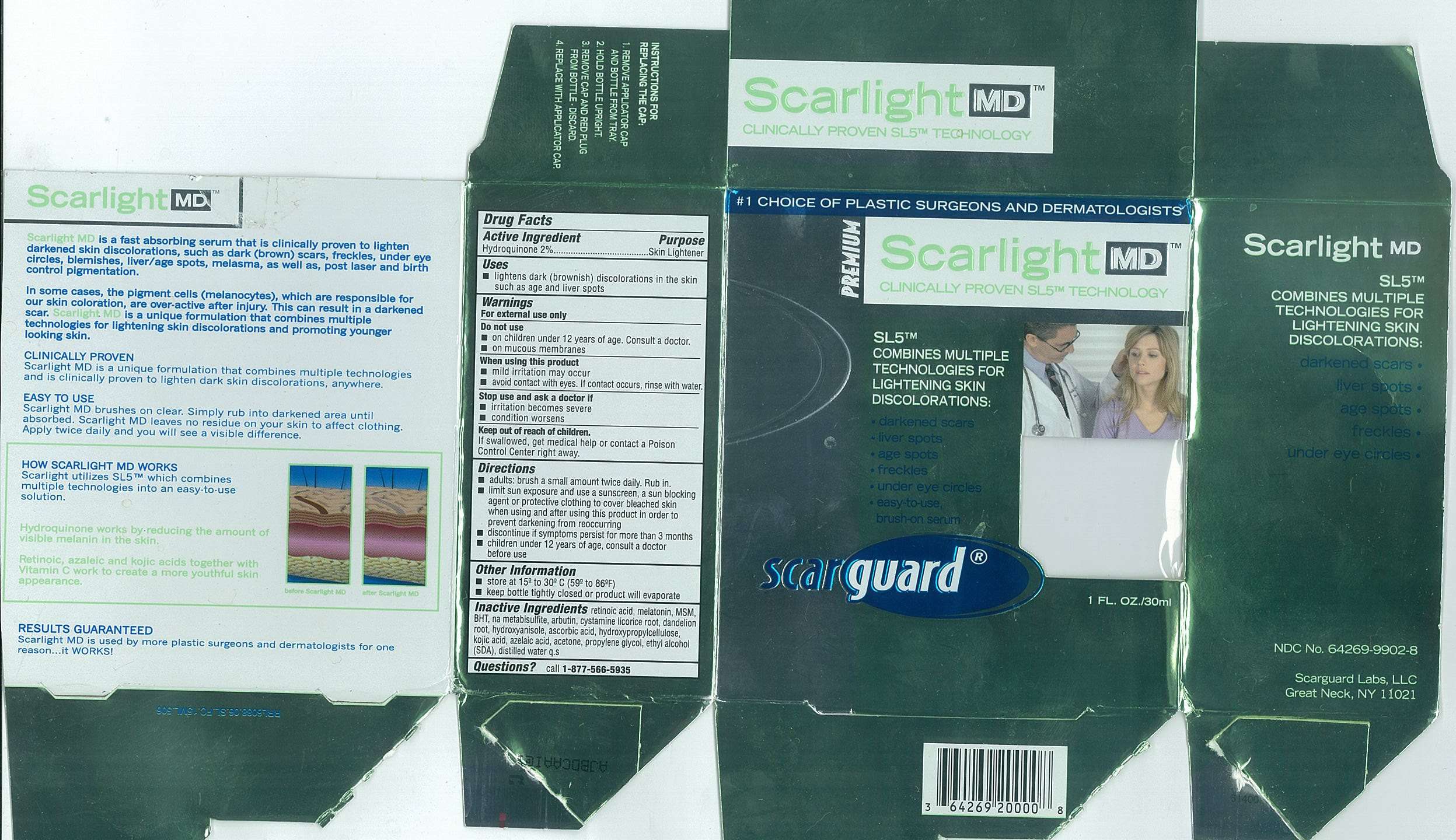
Scarlight MD
Scarguard Labs, LLC
Scarguard Labs, LLC
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Scarlight MD Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Other Information
- Inactive Ingredients
- Questions
- Carton 15mL
- Carton 30mL
FULL PRESCRIBING INFORMATION
Active Ingredients
Hydroquinone 2%
Purpose
Skin Lightener
Scarlight MD Uses
- lightens dark (brownish) discoloration in the skin such as age and liver spots
Warnings
For external use only
Do not use
- on children under 12 years of age. Consult a doctor.
- on mucous membranes
When using this product
- mild irritation may occur
- avoid contact with eyes. If contact occurs, rinse with water.
Stop use and ask a doctor if
- irritation becomes severe
- condition worsens
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults: brush a small amount twice daily. Rub in.
- limit sun exposure and use a sunscreen, a sun blocking agent or protective clothing to cover bleached skin when using and after using this product in order to prevent darkening from reoccurring
- discontinue if symptoms persist for more than 3 months
- children under 12 years of age, consult a doctor before use
Other Information
- store at 15º to 30ºC (59 to 86ºF)
- keep bottle tightly closed or product will evaporate
Inactive Ingredients
retinoic acid, melatonin, MSM, BHT, na metabisulfite, arbutin, cystamine, licorice root, dandelion root, hydroxyanisole, ascorbic acid, hydroxypropylcellulose, kojic acid, azelaic acid, acetone, propylene glycol, ethyl alcohol (SDA), distilled water q.s.
Questions
call
1-877-566-5935Carton 15mL
Carton 30mL
Scarlight MDHYDROQUINONE LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!