Selenium Sulfide
Morton Grove Pharmaceuticals, Inc.
SELENIUM SULFIDE LOTION, USP 2.5%
FULL PRESCRIBING INFORMATION: CONTENTS*
- APPLICATION INSTRUCTIONS
- WARNINGS AND PRECAUTIONS:
- CLINICAL PHARMACOLOGY:
- OVERDOSAGE:
- SELENIUM SULFIDE DOSAGE AND ADMINISTRATION:
- For treatment of tinea versicolor:
- For treatment of dandruff and seborrheic dermatitis:
- HOW SUPPLIED
- WARNINGS
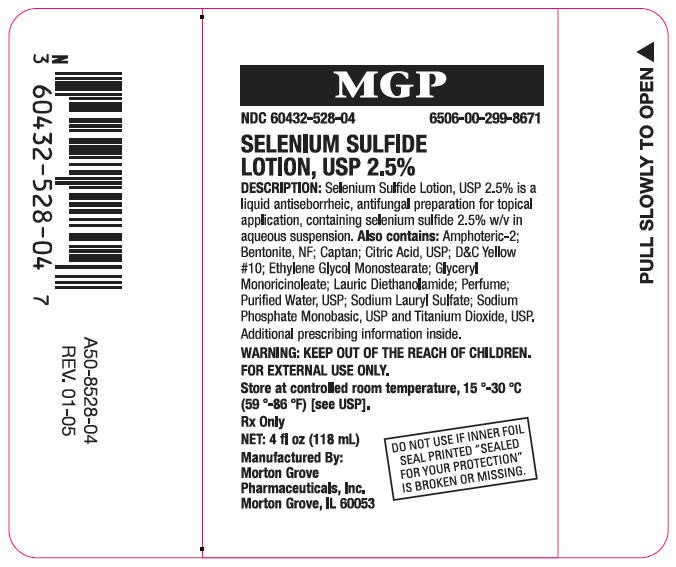
- PRINCIPAL DISPLAY PANEL - 118 mL Bottle Label
FULL PRESCRIBING INFORMATION
SHAKE WELL BEFORE USING.
APPLICATION INSTRUCTIONS
Keep tightly capped. SHAKE WELL BEFORE USING. Product may damage jewelry; remove jewelry before use.
For treatment of tinea versicolor:
- Apply to affected areas and lather with a small amount of water.
- Allow to remain on skin for 10 minutes.
- Rinse body thoroughly.
- Repeat this procedure once a day for 7 days.
For treatment of dandruff and seborrheic dermatitis of the scalp.
- Massage 1 or 2 teaspoonfuls of shampoo into wet scalp.
- Allow to remain on scalp for 2 to 3 minutes.
- Rinse scalp thoroughly.
- Repeat application and rinse thoroughly.
- After treatment, wash hands well.
- Repeat treatments as directed by physician.
WARNINGS AND PRECAUTIONS:
For External Use Only. Do not use on broken skin or inflamed areas. If allergic reactions occur, discontinue use. Avoid getting shampoo in eyes or in contact with genital area as it may cause irritation and burning.
FOR EXTERNAL USE ONLY. WARNING: KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Store at controlled room temperature, (15 - 30) °C ((59 - 86) °F) [see USP].
CLINICAL PHARMACOLOGY:
Selenium sulfide appears to have a cytostatic effect on cells of the epidermis and follicular epithelium, reducing corneocyte production.
INDICATIONS AND USAGE:
For treatment of tinea versicolor, seborrheic dermatitis of scalp and treatment of dandruff.
CONTRAINDICATIONS:
Not to be used by patients allergic to any of its ingredients.
PRECAUTIONS:
General:
Not to be used when acute inflammation or exudation is present as increased absorption may occur.
Information for Patients:
See Warnings and Precautions section under Application Instructions.
Carcinogenesis:
Dermal application of 25% and 50% solutions of 2.5% selenium sulfide lotion on mice over an 88 week period, indicated no carcinogenic effects.
Pregnancy:
WHEN USED ON BODY SURFACES FOR THE TREATMENT OF TINEA VERSICOLOR, SELENIUM SULFIDE LOTION, USP 2.5% IS CLASSIFIED AS PREGNANCY CATEGORY C. Animal reproduction studies have not been conducted with selenium sulfide. It is also not known whether selenium sulfide can cause fetal harm when applied to body surfaces of a pregnant woman or can affect reproduction capacity. Under ordinary circumstances selenium sulfide should not be used for the treatment of tinea versicolor in pregnant women.
Pediatric Use:
Safety and effectiveness in infants have not been established.
ADVERSE REACTIONS:
In decreasing order of severity: skin irritation; occasional reports of increase in normal hair loss; discoloration of hair (can be avoided or minimized by thorough rinsing of hair after treatment). As with other shampoos, oiliness or dryness of hair and scalp may occur.
OVERDOSAGE:
Accidental Oral Ingestion:
No documented reports of serious toxicity in humans resulting from acute ingestion of selenium sulfide, however, acute toxicity studies in animals suggest that ingestion of large amounts could result in potential human toxicity. Evacuation of the stomach contents should be considered in cases of acute oral ingestion.
DOSAGE AND ADMINISTRATION:
For treatment of tinea versicolor:
Apply to affected areas and lather with a small amount of water. Allow product to remain on skin for 10 minutes, then rinse the body thoroughly. Repeat procedure once a day for 7 days.
For treatment of dandruff and seborrheic dermatitis:
Usually two applications each week for two weeks will afford control. After this, the lotion may be used at less frequent intervals – weekly, every two weeks, or every 3 or 4 weeks in some cases. Should not be applied more frequently than required to maintain control.
HOW SUPPLIED
Selenium Sulfide Lotion, USP 2.5% is supplied in 4 fl oz (118 mL) bottles.
PROTECT FROM HEAT. FOR EXTERNAL USE ONLY.
WARNINGS
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN. In case of accidental overdose, seek professional assistance or contact a Poison Control Center immediately.
Rx Only
Product No.: 8528
A50-8528-04
REV. 01-05
Manufactured By:
Morton Grove Pharmaceuticals, Inc.
Morton Grove, IL 60053
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Label
MGP
NDC 60432-528-04
6506-00-299-8671
SELENIUM SULFIDE
LOTION, USP 2.5%
DESCRIPTION: Selenium Sulfide Lotion, USP 2.5% is a
liquid antiseborrheic, antifungal preparation for topical
application, containing selenium sulfide 2.5% w/v in
aqueous suspension. Also contains: Amphoteric-2;
Bentonite, NF; Captan; Citric Acid, USP; D&C Yellow
#10; Ethylene Glycol Monostearate; Glyceryl
Monoricinoleate; Lauric Diethanolamide; Perfume;
Purified Water, USP; Sodium Lauryl Sulfate; Sodium
Phosphate Monobasic, USP and Titanium Dioxide, USP.
Additional prescribing information inside.
WARNING: KEEP OUT OF THE REACH OF CHILDREN.
FOR EXTERNAL USE ONLY.
Store at controlled room temperature, (15 - 30) °C
((59 - 86) °F) [see USP].
Rx Only
NET: 4 fl oz (118 mL)
Manufactured By:
Morton Grove
Pharmaceuticals, Inc.
Morton Grove, IL 60053
DO NOT USE IF INNER FOIL
SEAL PRINTED "SEALED
FOR YOUR PROTECTION"
IS BROKEN OR MISSING.
Selenium SulfideSelenium Sulfide LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||