Sensorcaine
General Injectables & Vaccines, Inc
Sensorcaine 0.25% (Bupivacaine HCl) 50mL
FULL PRESCRIBING INFORMATION: CONTENTS*
- SENSORCAINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- SENSORCAINE CONTRAINDICATIONS
- PRECAUTIONS
- SENSORCAINE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- PACKAGE LABEL
FULL PRESCRIBING INFORMATION
SENSORCAINE DESCRIPTION
Sensorcaine® (bupivacaine HCl) injections are sterile isotonic solutions that contain a local anesthetic agent with and without
epinephrine (as bitartrate) 1:200,000 and are administered parenterally by injection. See INDICATIONS AND USAGE for specific
uses. Solutions of bupivacaine HCl may be autoclaved if they do not contain epinephrine.
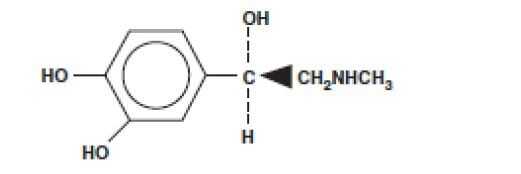
Sensorcaine injections contain bupivacaine HCl which is chemically designated as 2-piperidinecarboxamide, 1-butyl-N-(2, 6-
dimethylphenyl)-, monohydrochloride, monohydrate and has the following structure:

CLINICAL PHARMACOLOGY
Local anesthetics block the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical
excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential.
In general, the progression of anesthesia is related to the diameter, myelination and conduction velocity of affected nerve fibers.
Clinically, the order of loss of nerve function is as follows: (1) pain, (2) temperature, (3) touch, (4) proprioception, and (5) skeletal
muscle tone.
Systemic absorption of local anesthetics produces effects on the cardiovascular and central nervous systems. At blood concentrations
achieved with therapeutic doses, changes in cardiac conduction, excitability, refractoriness, contractility, and peripheral vascular
resistance are minimal. However, toxic blood concentrations depress cardiac conduction and excitability, which may lead to
atrioventricular block, ventricular arrhythmias and cardiac arrest, sometimes resulting in fatalities. In addition, myocardial
contractility is depressed and peripheral vasodilation occurs, leading to decreased cardiac output and arterial blood pressure. Recent
clinical reports and animal research suggest that these cardiovascular changes are more likely to occur after unintended intravascular
injection of bupivacaine. Therefore, incremental dosing is necessary.
Following systemic absorption, local anesthetics can produce central nervous system stimulation, depression or both. Apparent
central stimulation is usually manifested as restlessness, tremors and shivering progressing to convulsions, followed by depression and
coma progressing ultimately to respiratory arrest. However, the local anesthetics have a primary depressant effect on the medulla and
on higher centers. The depressed stage may occur without a prior excited stage.
Pharmacokinetics
The rate of systemic absorption of local anesthetics is dependent upon the total dose and concentration of drug administered, the route
of administration, the vascularity of the administration site, and the presence or absence of epinephrine in the anesthetic solution. A
dilute concentration of epinephrine (1:200,000 or 5 mcg/mL) usually reduces the rate of absorption and peak plasma concentration of
bupivacaine, permitting the use of moderately larger total doses and sometimes prolonging the duration of action.
The onset of action with bupivacaine is rapid and anesthesia is long-lasting. The duration of anesthesia is significantly longer with
bupivacaine than with any other commonly used local anesthetic. It has also been noted that there is a period of analgesia that persists
after the return of sensation, during which time the need for potent analgesics is reduced.
Local anesthetics are bound to plasma proteins in varying degrees. Generally, the lower the plasma concentration of drug, the higher
the percentage of drug bound to plasma proteins.
Local anesthetics appear to cross the placenta by passive diffusion. The rate and degree of diffusion is governed by: (1) the degree of
plasma protein binding, (2) the degree of ionization, and (3) the degree of lipid solubility. Fetal/maternal ratios of local anesthetics
appear to be inversely related to the degree of plasma protein binding, because only the free, unbound drug is available for placental
transfer. Bupivacaine, with a high protein binding capacity (95%), has a low fetal/maternal ratio (0.2 to 0.4). The extent of placental
transfer is also determined by the degree of ionization and lipid solubility of the drug. Lipid soluble, nonionized drugs readily enter
the fetal blood from the maternal circulation.
Depending upon the route of administration, local anesthetics are distributed to some extent to all body tissues, with high
concentrations found in highly perfused organs such as the liver, lungs, heart, and brain.
Pharmacokinetic studies on the plasma profile of bupivacaine after direct intravenous injection suggest a three-compartment open
model. The first compartment is represented by the rapid intravascular distribution of the drug. The second compartment represents
the equilibration of the drug throughout the highly perfused organs such as the brain, myocardium, lungs, kidneys, and liver. The third
compartment represents an equilibration of the drug with poorly perfused tissues, such as muscle and fat. The elimination of drug
from tissue depends largely upon the ability of binding sites in the circulation to carry it to the liver where it is metabolized.
After injection of Sensorcaine (bupivacaine HCl) for caudal, epidural or peripheral nerve block in man, peak levels of bupivacaine in
the blood are reached in 30 to 45 minutes, followed by a decline to insignificant levels during the next 3 to 6 hours.
Various pharmacokinetic parameters of the local anesthetics can be significantly altered by the presence of hepatic or renal disease,
addition of epinephrine, factors affecting urinary pH, renal blood flow, the route of drug administration, and the age of the patient.
The half-life of bupivacaine in adults is 2.7 hours and in neonates 8.1 hours.
In clinical studies, elderly patients reached the maximal spread of analgesia and maximal motor blockade more rapidly than younger
patients. Elderly patients also exhibited higher peak plasma concentrations following administration of this product. The total plasma
clearance was decreased in these patients.
Amide-type local anesthetics such as bupivacaine are metabolized primarily in the liver via conjugation with glucuronic acid.
Patients with hepatic disease, especially those with severe hepatic disease, may be more susceptible to the potential toxicities of the
amide-type local anesthetics. Pipecoloxylidine is the major metabolite of bupivacaine.
The kidney is the main excretory organ for most local anesthetics and their metabolites. Urinary excretion is affected by renal
perfusion and factors affecting urinary pH. Only 6% of bupivacaine is excreted unchanged in the urine.
When administered in recommended doses and concentrations, Sensorcaine (bupivacaine HCl) does not ordinarily produce irritation
or tissue damage and does not cause methemoglobinemia.
INDICATIONS & USAGE
Sensorcaine (bupivacaine HCl) is indicated for the production of local or regional anesthesia or analgesia for surgery, oral surgery
procedures, diagnostic and therapeutic procedures, and for obstetrical procedures. Only the 0.25% and 0.5% concentrations are
indicated for obstetrical anesthesia (see WARNINGS).
Experience with non-obstetrical surgical procedures in pregnant patients is not sufficient to recommend use of the 0.75%
concentration of bupivacaine HCl in these patients. Sensorcaine is not recommended for intravenous regional anesthesia (Bier Block)
(see WARNINGS).
The routes of administration and indicated Sensorcaine concentrations are:
local infiltration 0.25%
peripheral nerve block 0.25%, 0.5%
retrobulbar block 0.75%
sympathetic block 0.25%
lumbar epidural 0.25%, 0.5% and 0.75% (non-obstetrical)
caudal 0.25%, 0.5%
epidural test dose (see PRECAUTIONS)
(see DOSAGE AND ADMINISTRATION for additional information).
Standard textbooks should be consulted to determine the accepted procedures and techniques for the administration of Sensorcaine.
Use only the single dose ampules and single dose vials for caudal or epidural anesthesia; the multiple dose vials contain a preservative
and, therefore, should not be used for these procedures.
SENSORCAINE CONTRAINDICATIONS
Sensorcaine (bupivacaine HCl) is contraindicated in obstetrical paracervical block anesthesia. Its use by this technique has resulted in
fetal bradycardia and death.
Sensorcaine is contraindicated in patients with a known hypersensitivity to it or to any local anesthetic agent of the amide type or to
other components of bupivacaine solutions.
WARNINGS
THE 0.75% CONCENTRATION OF SENSORCAINE INJECTION IS NOT RECOMMENDED FOR OBSTETRICAL
ANESTHESIA. THERE HAVE BEEN REPORTS OF CARDIAC ARREST WITH DIFFICULT RESUSCITATION OR
DEATH DURING USE OF BUPIVACAINE FOR EPIDURAL ANESTHESIA IN OBSTETRICAL PATIENTS. IN MOST
CASES, THIS HAS FOLLOWED USE OF THE 0.75% CONCENTRATION. RESUSCITATION HAS BEEN DIFFICULT
OR IMPOSSIBLE DESPITE APPARENTLY ADEQUATE PREPARATION AND APPROPRIATE MANAGEMENT.
CARDIAC ARREST HAS OCCURRED AFTER CONVULSIONS RESULTING FROM SYSTEMIC TOXICITY,
PRESUMABLY FOLLOWING UNINTENTIONAL INTRAVASCULAR INJECTION. THE 0.75% CONCENTRATION
SHOULD BE RESERVED FOR SURGICAL PROCEDURES WHERE A HIGH DEGREE OF MUSCLE RELAXATION
AND PROLONGED EFFECT ARE NECESSARY.
LOCAL ANESTHETICS SHOULD ONLY BE EMPLOYED BY CLINICIANS WHO ARE WELL VERSED IN DIAGNOSIS
AND MANAGEMENT OF DOSE-RELATED TOXICITY AND OTHER ACUTE EMERGENCIES WHICH MIGHT ARISE
FROM THE BLOCK TO BE EMPLOYED, AND THEN ONLY AFTER INSURING THE IMMEDIATE AVAILABILITY
OF OXYGEN, OTHER RESUSCITATIVE DRUGS, CARDIOPULMONARY RESUSCITATIVE EQUIPMENT, AND
THE PERSONNEL RESOURCES NEEDED FOR PROPER MANAGEMENT OF TOXIC REACTIONS AND RELATED
EMERGENCIES (see also ADVERSE REACTIONS, PRECAUTIONS, and OVERDOSAGE). DELAY IN PROPER
MANAGEMENT OF DOSE-RELATED TOXICITY, UNDERVENTILATION FROM ANY CAUSE AND/OR ALTERED
SENSITIVITY MAY LEAD TO THE DEVELOPMENT OF ACIDOSIS, CARDIAC ARREST AND, POSSIBLY, DEATH.
Local anesthetic solutions containing antimicrobial preservatives, ie, those supplied in multiple dose vials, should not be used
for epidural or caudal anesthesia because safety has not been established with regard to intrathecal injection, either intentional or
unintentional, of such preservatives.
Intra-articular infusions of local anesthetics following arthroscopic and other surgical procedures is an unapproved use, and there have
been post-marketing reports of chondrolysis in patients receiving such infusions. The majority of reported cases of chondrolysis have
involved the shoulder joint; cases of gleno-humeral chondrolysis have been described in pediatric and adult patients following intraarticular
infusions of local anesthetics with and without epinephrine for periods of 48 to 72 hours. There is insufficient information to
determine whether shorter infusion periods are not associated with these findings. The time of onset of symptoms, such as joint pain,
stiffness and loss of motion can be variable, but may begin as early as the 2nd month after surgery. Currently, there is no effective
treatment for chondrolysis; patients who experienced chondrolysis have required additional diagnostic and therapeutic procedures and
some required arthroplasty or shoulder replacement.
It is essential that aspiration for blood or cerebrospinal fluid (where applicable) be done prior to injecting any local anesthetic, both
the original dose and all subsequent doses, to avoid intravascular or subarachnoid injection. However, a negative aspiration does not
ensure against an intravascular or subarachnoid injection.
Bupivacaine and Epinephrine Injection or other vasopressors should not be used concomitantly with ergot-type oxytocic drugs,
because a severe persistent hypertension may occur. Likewise, solutions of bupivacaine containing a vasoconstrictor, such as
epinephrine, should be used with extreme caution in patients receiving monoamine oxidase inhibitors (MAOI) or antidepressants of
the triptyline or imipramine types, because severe prolonged hypertension may result.
Until further experience is gained in pediatric patients younger than 12 years, administration of bupivacaine in this age group is not
recommended.
Mixing of the prior or intercurrent use of any local anesthetic with bupivacaine cannot be recommended because of insufficient data
on the clinical use of such mixtures.
There have been reports of cardiac arrest and death during the use of bupivacaine for intravenous regional anesthesia (Bier Block).
Information on safe dosages and techniques of administration of bupivacaine in this procedure is lacking. Therefore, bupivacaine is
not recommended for use in this technique.
Sensorcaine with epinephrine solutions contain sodium metabisulfite, a sulfite that may cause allergic-type reactions including
anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of
sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than
in nonasthmatic people.
Sensorcaine and Sensorcaine-MPF single dose vials do not contain sodium metabisulfite.
PRECAUTIONS
General
The safety and effectiveness of local anesthetics depend on proper dosage, correct technique, adequate precautions, and readiness
for emergencies. Resuscitative equipment, oxygen, and other resuscitative drugs should be available for immediate use (see
WARNINGS, ADVERSE REACTIONS, and OVERDOSAGE).
During major regional nerve blocks, the patient should have I.V. fluids running via an indwelling catheter to assure a functioning
intravenous pathway. The lowest dosage of local anesthetic that results in effective anesthesia should be used to avoid high plasma
levels and serious adverse effects. The rapid injection of a large volume of local anesthetic solution should be avoided and fractional
(incremental) doses should be used when feasible.
Epidural Anesthesia
During epidural administration of Sensorcaine (bupivacaine HCl), 0.5% and 0.75% solutions should be administered in incremental
doses of 3 to 5 mL with sufficient time between doses to detect toxic manifestations of unintentional intravascular or intrathecal
injection. Injections should be made slowly, with frequent aspirations before and during the injection to avoid intravascular injection.
Syringe aspirations should also be performed before and during each supplemental injection in continuous (intermittent) catheter
techniques. An intravascular injection is still possible even if aspirations for blood are negative.
During the administration of epidural anesthesia, it is recommended that a test dose be administered initially and the effects monitored
before the full dose is given. When using a “continuous” catheter technique, test doses should be given prior to both the original and
all reinforcing doses, because plastic tubing in the epidural space can migrate into a blood vessel or through the dura. When clinical
conditions permit, the test dose should contain epinephrine (10 to 15 mcg have been suggested) to serve as a warning of unintentional
intravascular injection. If injected into a blood vessel, this amount of epinephrine is likely to produce a transient “epinephrine
response” within 45 seconds, consisting of an increase in heart rate and systolic blood pressure, circumoral pallor, palpitations and
nervousness in the unsedated patient. The sedated patient may exhibit only a pulse rate increase of 20 or more beats per minute
for 15 or more seconds. Therefore, following the test dose, the heart rate should be monitored for a heart rate increase. Patients
on beta-blockers may not manifest changes in heart rate, but blood pressure monitoring can detect a transient rise in systolic blood
pressure. The test dose should also contain 10 mg to 15 mg of Sensorcaine or an equivalent amount of another local anesthetic to
detect an unintentional intrathecal administration. This will be evidenced within a few minutes by signs of spinal block (eg, decreased
sensation of the buttocks, paresis of the legs, or, in the sedated patient, absent knee jerk). An intravascular or subarachnoid injection
is still possible even if results of the test dose are negative. The test dose itself may produce a systemic toxic reaction, high spinal or
epinephrine-induced cardiovascular effects.
Injection of repeated doses of local anesthetics may cause significant increases in plasma levels with each repeated dose due to
slow accumulation of the drug or its metabolites or to slow metabolic degradation. Tolerance to elevated blood levels varies with
the physical condition of the patient. Debilitated, elderly patients, acutely ill patients and children should be given reduced doses
commensurate with their age and physical condition. Local anesthetics should also be used with caution in patients with hypotension
or heart block.
Careful and constant monitoring of cardiovascular and respiratory vital signs (adequacy of ventilation) and the patient’s state of
consciousness should be performed after each local anesthetic injection. It should be kept in mind at such times that restlessness,
anxiety, incoherent speech, light-headedness, numbness and tingling of the mouth and lips, metallic taste, tinnitus, dizziness, blurred
vision, tremors, twitching, depression, or drowsiness may be early warning signs of central nervous system toxicity.
Local anesthetic solutions containing a vasoconstrictor should be used cautiously and in carefully restricted quantities in areas of the
body supplied by end arteries or having otherwise compromised blood supply such as digits, nose, external ear, or penis. Patients with
hypertensive vascular disease may exhibit exaggerated vasoconstrictor response. Ischemic injury or necrosis may result.
Because amide-type local anesthetics such as bupivacaine are metabolized by the liver, these drugs, especially repeat doses, should
be used cautiously in patients with hepatic disease. Patients with severe hepatic disease, because of their inability to metabolize
local anesthetics normally, are at a greater risk of developing toxic plasma concentrations. Local anesthetics should also be used
with caution in patients with impaired cardiovascular function because they may be less able to compensate for functional changes
associated with the prolongation of A-V conduction produced by these drugs.
Serious dose-related cardiac arrhythmias may occur if preparations containing a vasoconstrictor such as epinephrine are employed
in patients during or following the administration of potent inhalation anesthetics. In deciding whether to use these products
concurrently in the same patient, the combined action of both agents upon the myocardium, the concentration and volume of
vasoconstrictor used, and the time since injection, when applicable, should be taken into account.
Many drugs used during the conduct of anesthesia are considered potential triggering agents for familial malignant hyperthermia.
Because it is not known whether amide-type local anesthetics may trigger this reaction and because the need for supplemental
general anesthesia cannot be predicted in advance, it is suggested that a standard protocol for management should be available.
Early unexplained signs of tachycardia, tachypnea, labile blood pressure and metabolic acidosis may precede temperature elevation.
Successful outcome is dependent on early diagnosis, prompt discontinuance of the suspect triggering agent(s) and prompt treatment,
including oxygen therapy, indicated supportive measures and dantrolene (consult dantrolene sodium intravenous package insert before
using).
Use in Head and Neck Area
Small doses of local anesthetics injected into the head and neck area, including retrobulbar, dental and stellate ganglion blocks, may
produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. The injection
procedures require the utmost care. Confusion, convulsions, respiratory depression, and/or respiratory arrest, and cardiovascular
stimulation or depression have been reported. These reactions may be due to intra-arterial injection of the local anesthetic with
retrograde flow to the cerebral circulation. They may also be due to puncture of the dural sheath of the optic nerve during retrobulbar
block with diffusion of any local anesthetic along the subdural space to the midbrain. Patients receiving these blocks should
have their circulation and respiration monitored and be constantly observed. Resuscitative equipment and personnel for treating
adverse reactions should be immediately available. Dosage recommendations should not be exceeded (see DOSAGE AND
ADMINISTRATION).
Use in Ophthalmic Surgery
Clinicians who perform retrobulbar blocks should be aware that there have been reports of respiratory arrest following local anesthetic
injection. Prior to retrobulbar block, as with all other regional procedures, the immediate availability of equipment, drugs, and
personnel to manage respiratory arrest or depression, convulsions, and cardiac stimulation or depression should be assured (see
also WARNINGS and Use in Head and Neck Area, above). As with other anesthetic procedures, patients should be constantly
monitored following ophthalmic blocks for signs of these adverse reactions, which may occur following relatively low total doses.
A concentration of 0.75% bupivacaine is indicated for retrobulbar block; however, this concentration is not indicated for any
other peripheral nerve block, including the facial nerve and not indicated for local infiltration, including the conjunctiva (see
INDICATIONS and PRECAUTIONS, General). Mixing Sensorcaine (bupivacaine HCl) with other local anesthetics is not
recommended because of insufficient data on the clinical use of such mixtures.
When Sensorcaine (bupivacaine HCl) 0.75% is used for retrobulbar block, complete corneal anesthesia usually precedes onset of
clinically acceptable external ocular muscle akinesia. Therefore, presence of akinesia rather than anesthesia alone should determine
readiness of the patient for surgery.
Information for Patients
When appropriate, patients should be informed in advance that they may experience temporary loss of sensation and motor activity,
usually in the lower half of the body following proper administration of caudal or lumbar epidural anesthesia. Also, when appropriate,
the physician should discuss other information including adverse reactions in the Sensorcaine package insert.
Clinically Significant Drug Interactions
The administration of local anesthetic solutions containing epinephrine or norepinephrine to patients receiving monoamine oxidase
inhibitors or tricyclic antidepressants may produce severe, prolonged hypertension. Concurrent use of these agents should generally
be avoided. In situations in which concurrent therapy is necessary, careful patient monitoring is essential.
Concurrent administration of vasopressor drugs and of ergot-type oxytocic drugs may cause severe, persistent hypertension or
cerebrovascular accidents.
Phenothiazines and butyrophenones may reduce or reverse the pressor effect of epinephrine.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term studies in animals of most local anesthetics, including bupivacaine, to evaluate the carcinogenic potential have not been
conducted. Mutagenic potential or the effect on fertility has not been determined. There is no evidence from human data that
Sensorcaine (bupivacaine HCl) may be carcinogenic or mutagenic or that it impairs fertility.
Pregnancy Category C
Decreased pup survival in rats and embryocidal effect in rabbits have been observed when bupivacaine HCl was administered to these
species in doses comparable to nine and five times, respectively, the maximum recommended daily human dose (400 mg). There
are no adequate and well-controlled studies in pregnant women of the effect of bupivacaine on the developing fetus. Sensorcaine
should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. This does not exclude the use of
Sensorcaine at term for obstetrical anesthesia or analgesia (see Labor and Delivery).
Labor and Delivery
SEE BOX WARNINGS REGARDING OBSTETRICAL USE IN 0.75% CONCENTRATION.
Sensorcaine is contraindicated in obstetrical paracervical block anesthesia.
Local anesthetics rapidly cross the placenta, and when used for epidural, caudal or pudendal block anesthesia, can cause varying
degrees of maternal, fetal and neonatal toxicity (see Pharmacokinetics in CLINICAL PHARMACOLOGY). The incidence and
degree of toxicity depend upon the procedure performed, the type and amount of drug used, and the technique of drug administration.
SENSORCAINE ADVERSE REACTIONS
Reactions to Sensorcaine (bupivacaine HCl) are characteristic of those associated with other amide-type local anesthetics. A
major cause of adverse reactions to this group of drugs is excessive plasma levels, which may be due to overdosage, unintentional
intravascular injection or slow metabolic degradation.
Systemic
The most commonly encountered acute adverse experiences that demand immediate countermeasures are related to the central nervous
system and the cardiovascular system. These adverse experiences are generally dose related and due to high plasma levels which may
result from overdosage, rapid absorption from the injection site, diminished tolerance or from unintentional intravascular injection
of the local anesthetic solution. In addition to systemic dose-related toxicity, unintentional subarachnoid injection of drug during
the intended performance of caudal or lumbar epidural block or nerve blocks near the vertebral column (especially in the head and
neck region) may result in underventilation or apnea (“Total or High Spinal”). Also, hypotension due to loss of sympathetic tone
and respiratory paralysis or underventilation due to cephalad extension of the motor level of anesthesia may occur. This may lead
to secondary cardiac arrest if untreated. Patients over 65 years, particularly those with hypertension, may be at increased risk for
experiencing the hypotensive effects of bupivacaine. Factors influencing plasma protein binding, such as acidosis, systemic diseases
that alter protein production or competition with other drugs for protein binding sites, may diminish individual tolerance.
Central Nervous System Reactions
These are characterized by excitation and/or depression. Restlessness, anxiety, dizziness, tinnitus, blurred vision or tremors
may occur, possibly proceeding to convulsions. However, excitement may be transient or absent, with depression being the first
manifestation of an adverse reaction. This may quickly be followed by drowsiness merging into unconsciousness and respiratory
arrest. Other central nervous system effects may be nausea, vomiting, chills, and constriction of the pupils.
OVERDOSAGE
Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local
anesthetics or to unintended subarachnoid injection of local anesthetic solution (see ADVERSE REACTIONS, WARNINGS, and
PRECAUTIONS).
Management of Local Anesthetic Emergencies
The first consideration is prevention, best accomplished by careful and constant monitoring of cardiovascular and respiratory vital
signs and the patient’s state of consciousness after each local anesthetic injection. At the first sign of change, oxygen should be
administered.
The first step in the management of systemic toxic reactions, as well as underventilation or apnea due to unintentional subarachnoid
injection of drug solution, consists of immediate attention to the establishment and maintenance of a patent airway and effective
assisted or controlled ventilation with 100% oxygen with a delivery system capable of permitting immediate positive airway pressure
by mask.This may prevent convulsions if they have not already occurred.
If necessary, use drugs to control the convulsions. A 50 to 100 mg bolus I.V. injection of succinylcholine will paralyze the patient
without depressing the central nervous or cardiovascular systems and facilitate ventilation. A bolus I.V. dose of 5 to 10 mg of
diazepam or 50 to 100 mg of thiopental will permit ventilation and counteract central nervous system stimulation, but these drugs also
depress the central nervous system, respiratory and cardiac function, add to postictal depression, and may result in apnea. Intravenous
barbiturates, anticonvulsant agents, or muscle relaxants should only be administered by those familiar with their use. Immediately
after the institution of these ventilatory measures, the adequacy of the circulation should be evaluated. Supportive treatment of
circulatory depression may require administration of intravenous fluids, and, when appropriate, a vasopressor dictated by the clinical
situation (such as ephedrine or epinephrine to enhance myocardial contractile force).
Endotracheal intubation, employing drugs and techniques familiar to the clinician, may be indicated after initial administration of
oxygen by mask, if difficulty is encountered in the maintenance of a patent airway or if prolonged ventilatory support (assisted or
controlled) is indicated.
Recent clinical data from patients experiencing local anesthetic-induced convulsions demonstrated rapid development of hypoxia,
hypercarbia, and acidosis with bupivacaine within a minute of the onset of convulsions. These observations suggest that oxygen
DOSAGE & ADMINISTRATION
The dose of any local anesthetic administered varies with the anesthetic procedure, the area to be anesthetized, the vascularity of the
tissues, the number of neuronal segments to be blocked, the depth of anesthesia and degree of muscle relaxation required, the duration
of anesthesia desired, individual tolerance, and the physical condition of the patient. The smallest dose and concentration required to
produce the desired result should be administered. Dosages of Sensorcaine should be reduced for young, elderly and/or debilitated
patients and patients with cardiac and/or liver disease. The rapid injection of a large volume of local anesthetic solution should be
avoided and fractional (incremental) doses should be used when feasible.
For specific techniques and procedures, refer to standard textbooks.
There have been adverse event reports of chondrolysis in patients receiving intra-articular infusions of local anesthetics following
arthroscopic and other surgical procedures. Sensorcaine is not approved for this use (see WARNINGS and DOSAGE AND
ADMINISTRATION).).
In recommended doses, Sensorcaine (bupivacaine HCl) produces complete sensory block, but the effect on motor function differs
among the three concentrations.
0.25%—when used for caudal, epidural, or peripheral nerve block, produces incomplete motor block. Should be used for operations
in which muscle relaxation is not important, or when another means of providing muscle relaxation is used concurrently. Onset of
action may be slower than with the 0.5% or 0.75% solutions.
0.5%—provides motor blockade for caudal, epidural, or nerve block, but muscle relaxation may be inadequate for operations in which
complete muscle relaxation is essential.
0.75%—produces complete motor block. Most useful for epidural block in abdominal operations requiring complete muscle
relaxation, and for retrobulbar anesthesia. Not for obstetrical anesthesia.
The duration of anesthesia with Sensorcaine is such that for most indications, a single dose is sufficient.
Maximum dosage limit must be individualized in each case after evaluating the size and physical status of the patient, as well as the
usual rate of systemic absorption from a particular injection site. Most experience to date is with single doses of Sensorcaine up to
225 mg with epinephrine 1:200,000 and 175 mg without epinephrine; more or less drug may be used depending on individualization
of each case.
These doses may be repeated up to once every three hours. In clinical studies to date, total daily doses up to 400 mg have been
reported. Until further experience is gained, this dose should not be exceeded in 24 hours. The duration of anesthetic effect may be
prolonged by the addition of epinephrine.
The dosages in Table 1 have generally proved satisfactory and are recommended as a guide for use in the average adult. These
dosages should be reduced for elderly or debilitated patients. Until further experience is gained Sensorcaine is not recommended for
pediatric patients younger than 12 years. Sensorcaine is contraindicated for obstetrical paracervical blocks, and is not recommended
for intravenous regional anesthesia (Bier Block).
Use in Epidural Anesthesia
During epidural administration of Sensorcaine, 0.5% and 0.75% solutions should be administered in incremental doses of 3 mL to
5 mL with sufficient time between doses to detect toxic manifestations of unintentional intravascular or intrathecal injection. In
obstetrics, only the 0.5% and 0.25% concentrations should be used; incremental doses of 3 mL to 5 mL of the 0.5% solution not
exceeding 50 mg to 100 mg at any dosing interval are recommended. Repeat doses should be preceded by a test dose containing
epinephrine if not contraindicated. Use only the single dose ampules and single dose vials for caudal or epidural anesthesia; the
multiple dose vials contain a preservative and therefore should not be used for these procedures.
Test Dose for Caudal and Lumbar Epidural Blocks
See PRECAUTIONS.
Unused portions of solutions in single dose containers should be discarded, since this product form contains no preservatives.
TABLE 1. DOSAGE RECOMMENDATIONS —
SENSORCAINE (bupivacaine HCl) INJECTIONS
|
|
|
Each Dose |
Each Dose |
|
| Type of Block |
Conc |
(mL) |
(mg) |
Motor Block (1) |
| Local Infiltration |
0.25% (4) |
up to max. |
up to max. |
_ |
| Epidural |
0.75% (2,4) |
10 to 20 |
75 to 150 |
complete |
|
|
0.5% (4) |
10 to 20 |
50 to 100 |
moderate to complete |
|
|
0.25% (4) |
10 to 20 |
25 to 50 |
partial to moderate |
| Caudal |
0.5% (4) |
15 to 30 |
75 to 150 |
moderate to complete |
|
|
0.25% (4) |
15 to 30 |
37.5 to 75 |
moderate |
| Peripheral Nerves |
0.5% (4) |
5 to max. |
25 to max. |
moderate to complete |
|
|
0.25% (4) |
5 to max. |
12.5 to max. |
moderate to complete |
| Retrobulbar (3) |
0.75% (4) |
2 to 4 |
15 to 30 |
complete |
| Sympathetic |
0.25% |
20 to 50 |
50 to 125 |
_ |
| Epidural (3) |
0.5% |
2 to 3 |
10 to 15 |
_ |
| Test Dose |
w/epi |
|
10 to 15 mcg epinephrine (see PRECAUTIONS) |
10 to 15 mcg epinephrine (see PRECAUTIONS) |
| Product No. |
NDC No. |
Strength |
Size |
| 460837 |
63323-468-37 |
0.25% |
30 mL Single Dose Vials packaged in trays of 25. |
| 460817 |
63323-468-17 |
0.25% |
10 mL Single Dose Vials packaged in trays of 25. |
| 460217 |
63323-462-17 |
0.5% |
10 mL Single Dose Vials packaged in trays of 25. |
| 460237 |
63323-462-37 |
0.5% |
30 mL Single Dose Vials packaged in trays of 25. |
| 460231 |
63323-462-31 |
0.5% |
30 mL Single Dose Vials packaged in 5. |
| 461037 |
63323-460-37 |
0.75% |
30 mL Single Dose Vials packaged in trays of 25. |
| Product No. |
NDC No. |
Strength |
Size |
| 460417 |
63323-464-17 |
0.25% |
10 mL Single Dose Vials packaged in trays of 25. |
| 460433* |
63323-464-33 |
0.25% |
30 mL ampules packaged in 5. |
| 460437 |
63323-464-37 |
0.25% |
30 mL Single Dose Vials packaged in trays of 25. |
| 460431 |
63323-464-31 |
0.25% |
30 mL Single Dose Vials packaged in 5. |
| 460617 |
63323-466-17 |
0.5% |
10 mL Single Dose Vials packaged in trays of 25. |
| 460637 |
63323-466-37 |
0.5% |
30 mL Single Dose Vials packaged in trays of 25. |
| 460631 |
63323-466-31 |
0.5% |
30 mL Single Dose Vials packaged in 5. |
| 460633* |
63323-466-33 |
0.5% |
30 mL ampules packaged in 5. |
| 470217 |
63323-472-17 |
0.75% |
10 mL Single Dose Vials packaged in trays of 25. |
| 470237 |
63323-472-37 |
0.75% |
30 mL Single Dose Vials packaged in trays of 25. |
| 470233* |
63323-472-33 |
0.75% |
30 mL ampules packaged in 5. |
| Product No. |
NDC No. |
Strength |
Size |
| 460157 |
63323-461-57 |
0.25% |
50 mL Multiple Dose Vials packaged in trays of 25. |
| 460357 |
63323-463-57 |
0.5% |
50 mL Multiple Dose Vials packaged in trays of 25 |
| Product No. |
NDC No. |
Strength |
Size |
| 460557 |
63323-465-57 |
0.25% |
50 mL Multiple Dose Vials packaged in trays of 25. |
| 460757 |
63323-467-57 |
0.5% |
50 mL Multiple Dose Vials packaged in trays of 25. |
PACKAGE LABEL

SensorcaineBupivacaine Hydrochloride INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||