General Injectables & Vaccines, Inc
Sensorcaine -MPF (Bupivacaine HCl Injection, USP) 0.5% 10mL single dose vial
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
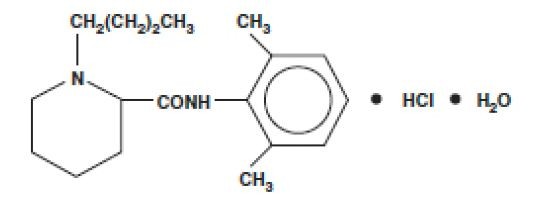
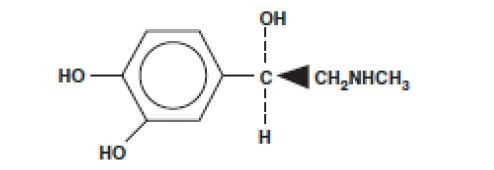
SENSORCAINE MPF DESCRIPTION
CLINICAL PHARMACOLOGY
Local anesthetics block the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. In general, the progression of anesthesia is related to the diameter, myelination and conduction velocity of affected nerve fibers. Clinically, the order of loss of nerve function is as follows: (1) pain, (2) temperature, (3) touch, (4) proprioception, and (5) skeletal muscle tone.
Systemic absorption of local anesthetics produces effects on the cardiovascular and central nervous systems. At blood concentrations achieved with therapeutic doses, changes in cardiac conduction, excitability, refractoriness, contractility, and peripheral vascular resistance are minimal. However, toxic blood concentrations depress cardiac conduction and excitability, which may lead to atrioventricular block, ventricular arrhythmias and cardiac arrest, sometimes resulting in fatalities. In addition, myocardial contractility is depressed and peripheral vasodilation occurs, leading to decreased cardiac output and arterial blood pressure. Recent clinical reports and animal research suggest that these cardiovascular changes are more likely to occur after unintended intravascular injection of bupivacaine. Therefore, incremental dosing is necessary.
Following systemic absorption, local anesthetics can produce central nervous system stimulation, depression or both. Apparent central stimulation is usually manifested as restlessness, tremors and shivering progressing to convulsions, followed by depression and coma progressing ultimately to respiratory arrest. However, the local anesthetics have a primary depressant effect on the medulla andon higher centers. The depressed stage may occur without a prior excited stage.
INDICATIONS & USAGE
SENSORCAINE MPF CONTRAINDICATIONS
PRECAUTIONS
SENSORCAINE MPF ADVERSE REACTIONS
Reactions to Sensorcaine (bupivacaine HCl) are characteristic of those associated with other amide-type local anesthetics. A major cause of adverse reactions to this group of drugs is excessive plasma levels, which may be due to overdosage, unintentional intravascular injection or slow metabolic degradation.
Systemic
The most commonly encountered acute adverse experiences that demand immediate countermeasures are related to the central nervous system and the cardiovascular system. These adverse experiences are generally dose related and due to high plasma levels which may result from overdosage, rapid absorption from the injection site, diminished tolerance or from unintentional intravascular injection of the local anesthetic solution. In addition to systemic dose-related toxicity, unintentional subarachnoid injection of drug during the intended performance of caudal or lumbar epidural block or nerve blocks near the vertebral column (especially in the head and neck region) may result in underventilation or apnea (“Total or High Spinal”). Also, hypotension due to loss of sympathetic tone and respiratory paralysis or underventilation due to cephalad extension of the motor level of anesthesia may occur. This may lead to secondary cardiac arrest if untreated. Patients over 65 years, particularly those with hypertension, may be at increased risk for experiencing the hypotensive effects of bupivacaine. Factors influencing plasma protein binding, such as acidosis, systemic diseases that alter protein production or competition with other drugs for protein binding sites, may diminish individual tolerance.
Central Nervous System Reactions
These are characterized by excitation and/or depression. Restlessness, anxiety, dizziness, tinnitus, blurred vision or tremors may occur, possibly proceeding to convulsions. However, excitement may be transient or absent, with depression being the first manifestation of an adverse reaction. This may quickly be followed by drowsiness merging into unconsciousness and respiratory arrest. Other central nervous system effects may be nausea, vomiting, chills, and constriction of the pupils. The incidence of convulsions associated with the use of local anesthetics varies with the procedure used and the total dose administered. In a survey of studies of epidural anesthesia, overt toxicity progressing to convulsions occurred in approximately 0.1% of local anesthetic administrations.
Cardiovascular System Reactions
OVERDOSAGE
DOSAGE & ADMINISTRATION
|
|
Each Dose
|
|
|
Type of Block
|
Conc.
|
(mL)
|
(mg)
|
Motor Block (1)
|
Local Infiltration
|
0.25% (4)
|
up to max.
|
up to max.
|
_
|
Epidural
|
0.75% (2,4)
|
10 to 20
|
75 to 150
|
complete
|
|
0.5% (4)
|
10 to 20
|
50 to 100
|
moderate to complete
|
|
0.25% (4)
|
10 to 20
|
25 to 50
|
partial to moderate
|
Caudal
|
0.5% (4)
|
15 to 30
|
75 to 150
|
moderate to complete
|
|
0.25% (4)
|
15 to 30
|
37.5 to 75
|
moderate
|
Peripheral Nerves
|
0.5% (4)
|
5 to max.
|
25 to max.
|
moderate to complete
|
|
0.25% (4)
|
5 to max.
|
12.5 to max.
|
moderate to complete
|
Retrobulbar (3)
|
0.75% (4)
|
2 to 4
|
15 to 30
|
complete
|
Sympathetic
|
0.25%
|
20 to 50
|
50 to 125
|
_
|
Epidural (3)
|
0.5%
|
2 to 3
|
10 to 15
|
_
|
Test Dose
|
w/epi
|
|
10 to 15 mcg epinephrine (see PRECAUTIONS)
|
10 to 15 mcg epinephrine (see PRECAUTIONS) |
HOW SUPPLIED
Product No.
|
NDC No.
|
Strength
|
Size
|
460837
|
63323-468-37
|
0.25%
|
30mL Single Dose Vials packaged in trays of 25.
|
460817
|
63323-468-17
|
0.25%
|
10mL Single Dose Vials packaged in trays of 25.
|
460217
|
63323-462-17
|
0.5%
|
10mL Single Dose Vials packaged in trays of 25.
|
460237
|
63323-462-37
|
0.5%
|
30mL Single Dose Vials packaged in trays of 25.
|
460231
|
63323-462-31
|
0.5%
|
30mL Single Dose Vials packaged in 5.
|
461037
|
63323-460-37
|
0.75%
|
30mL Single Dose Vials packaged in trays of 25.
|
Product No.
|
NDC No.
|
Strength
|
Size
|
460417
|
63323-464-17
|
0.25%
|
10 mL Single Dose Vials packaged in trays of 25
|
460433*
|
63323-464-33
|
0.25%
|
30 mL ampules packaged in 5
|
460437
|
63323-464-37
|
0.25%
|
30 mL Single Dose Vials packaged in trays of 25
|
460431
|
63323-464-31
|
0.25%
|
30 mL Single Dose Vials packaged in 5
|
460617
|
63323-466-17
|
0.5%
|
10 mL Single Dose Vials packaged in trays of 25
|
460637
|
63323-466-37
|
0.5%
|
30 mL Single Dose Vials packaged in trays of 25
|
460631
|
63323-466-31
|
0.5%
|
30 mL Single Dose Vials packaged in 5
|
460633*
|
63323-466-33
|
0.5%
|
30 mL ampules packaged in 5
|
470217
|
63323-472-17
|
0.75%
|
10 mL Single Dose Vials packaged in trays of 25
|
470237
|
63323-472-37
|
0.75%
|
30 mL Single Dose Vials packaged in trays of 25
|
470233*
|
63323-472-33
|
0.75%
|
30 mL ampules packaged in 5
|
Product No.
|
NDC No.
|
Strength
|
Size
|
460157
|
63323-461-57
|
0.25%
|
50 mL Multiple Dose Vials packaged in trays of 25
|
460357
|
63323-463-57
|
0.5%
|
50 mL Multiple Dose Vials packaged in trays of 25
|
Product No.
|
NDC No.
|
Strength
|
Size
|
460557
|
63323-465-57
|
0.25%
|
50 mL Multiple Dose Vials packaged in trays of 25
|
460757
|
63323-467-57
|
0.5%
|
50 mL Multiple Dose Vials packaged in trays of 25
|
PACKAGE LABEL

Sensorcaine MPF
bupivacaine hydrochloride INJECTION, SOLUTION
Product Information
|
|
Product Type
|
Human prescription drug label |
Item Code (Source)
|
NDC:52584-466(NDC:63323-466) |
|
Route of Administration
|
PERINEURAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
10 in 1 VIAL |
|
|
|
2 |
NDC:52584-466-17 |
1 in 1 BAG |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
ANDA |
ANDA070553 |
2011-10-26 |
|
|


