Sertraline Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- SERTRALINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- INDICATIONS & USAGE
- SERTRALINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- SERTRALINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Suicidality and Antidepressant DrugsAntidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of Sertraline Hydrochloride Tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Sertraline Hydrochloride Tablets are not approved for the treatment of major depressive disorder in pediatric patients. (SeeWarnings: Clinical Worsening and Suicide Risk,Precautions: Information for Patients, andPrecautions: Pediatric Use)
SERTRALINE HYDROCHLORIDE DESCRIPTION
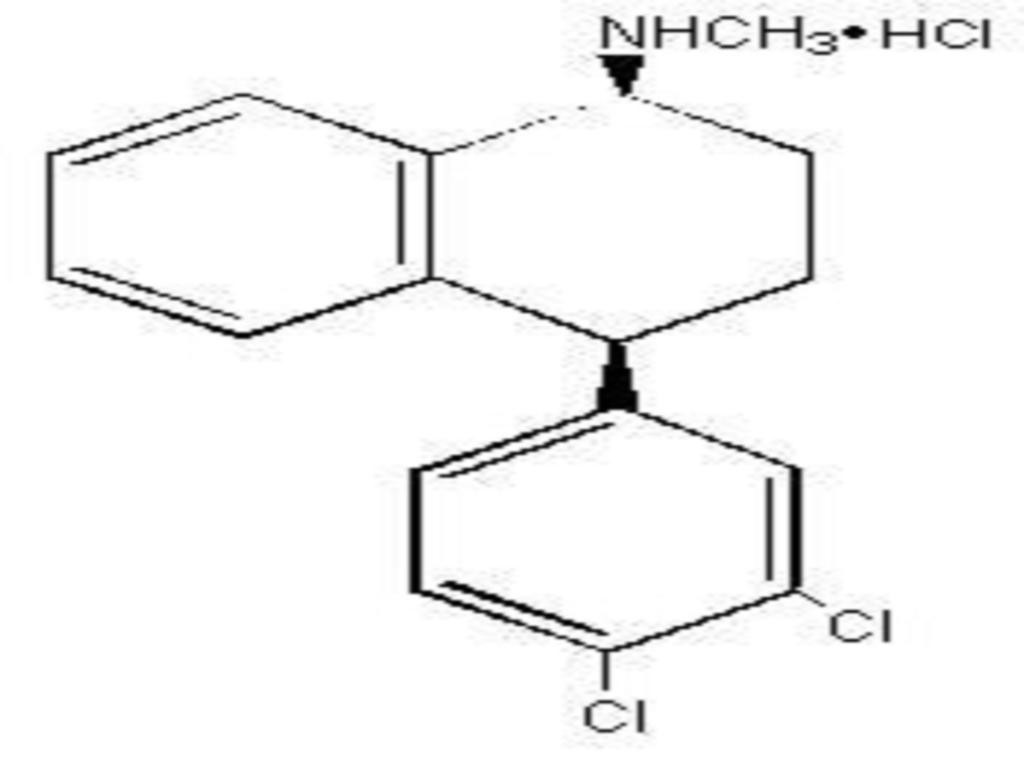
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS
PHARMACOKINETICS
Systemic BioavailabilityMetabolism
Protein Binding
PRECAUTIONS
Pediatric Pharmacokinetics
DOSAGE AND ADMINISTRATION
Age
Liver Disease
PRECAUTIONSDOSAGE AND ADMINISTRATION
Renal Disease
PRECAUTIONS
Clinical Trials
Major Depressive Disorder
Premenstrual Dysphoric Disorder (PMDD)
INDICATIONS & USAGE
Major Depressive DisorderClinical Trials under CLINICAL PHARMACOLOGY
Clinical Trials under CLINICAL PHARMACOLOGY
Premenstrual Dysphoric Disorder (PMDD)
Clinical Trials under CLINICAL PHARMACOLOGY
DOSAGE AND ADMINISTRATION).
SERTRALINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGSPRECAUTIONSWARNINGS
Clinical Worsening and Suicide RiskAge RangeDrug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated
PRECAUTIONSDOSAGE AND ADMINISTRATIONDiscontinuation of Treatment
Screening Patients for Bipolar Disorder
CONTRAINDICATIONSWARNINGSPotential for Interaction with Monoamine Oxidase Inhibitors
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)- like Reactions:
PRECAUTIONS
GeneralActivation of Mania/Hypomania
Weight Loss
Seizure
Discontinuation of Treatment with Sertraline
DOSAGE AND ADMINISTRATION
Abnormal Bleeding
Weak Uricosuric Effect
Use in Patients with Concomitant Illness
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
CLINICAL PHARMACOLOGY
Interference with Cognitive and Motor Performance
Information for Patients
Hyponatremia
GERIATRIC USE
Platelet Function
INFORMATION FOR PATIENTS
Clinical Worsening and Suicide Risk
LABORATORY TESTS
DRUG INTERACTIONS
Potential Effects of Coadministration of Drugs Highly Bound to Plasma ProteinsCimetidine
CNS Active Drugs
CONTRAINDICATIONS
Monoamine Oxidase Inhibitors
CONTRAINDICATIONSWARNINGS
Drugs Metabolized by P450 3A4
Drugs Metabolized by P450 2D6
Tricyclic Antidepressant Drugs Effective in the Treatment of Major Depressive Disorder under PRECAUTIONS
Serotonergic Drugs
WARNINGS-Serotonin SyndromePRECAUTIONSDrug Interactions
Triptans
WARNINGSSerotonin Syndrome
Sumatriptan
Tricyclic Antidepressant Drugs Effective in the Treatment of Major Depressive Disorder (TCAs)
Drugs Metabolized by P450 2D6 under PRECAUTIONS
Hypoglycemic Drugs
Atenolol
Digoxin
Microsomal Enzyme Induction
Drugs That Interfere With Hemostasis (Non-selective NSAIDs, Aspirin, Warfarin, etc.)
Electroconvulsive Therapy
Alcohol
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CarcinogenesisMutagenesis
Impairment of Fertility
PREGNANCY
Pregnancy Category CPregnancy-Nonteratogenic Effects
WARNINGS
DOSAGE AND ADMINISTRATION
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
BOX WARNINGWARNINGSClinical Worsening and Suicide RiskPharmacokineticsCLINICAL PHARMACOLOGY
ADVERSE REACTIONS
WARNINGSClinical Worsening and Suicide Risk
GERIATRIC USE
ADVERSE REACTIONSPRECAUTIONS, Hyponatremia
SERTRALINE HYDROCHLORIDE ADVERSE REACTIONS
Incidence in Placebo-Controlled Trials
Associated with Discontinuation in Placebo-Controlled Clinical Trials
Male and Female Sexual Dysfunction with SSRIs
Other Adverse Events in Pediatric Patients
Other Events Observed During the Premarketing Evaluation of Sertraline Hydrochloride
PRECAUTIONS
OVERDOSAGE
Human ExperienceOverdose Management
DOSAGE & ADMINISTRATION
Initial TreatmentDosage for Adults
Major Depressive Disorder and Obsessive-Compulsive Disorder
Premenstrual Dysphoric Disorder
Clinical Trials under CLINICAL PHARMACOLOGY
Maintenance/Continuation/Extended Treatment
Major Depressive Disorder
Clinical Trials under CLINICAL PHARMACOLOGY
Premenstrual Dysphoric Disorder
Switching Patients to or from a Monoamine Oxidase Inhibitor
CONTRAINDICATIONSWARNINGS
Special Populations
Dosage for Hepatically Impaired Patients
CLINICAL PHARMACOLOGYPRECAUTIONS
Treatment of Pregnant Women During the Third Trimester
PRECAUTIONS
Discontinuation of Treatment with Sertraline
PRECAUTIONS
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Sertraline HydrochlorideSertraline Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!