SHISEIDO WHITE LUCENT
SHISEIDO WHITE LUCENTBRIGHTENING PROTECTIVE CREAM WSPF 15 • PA++
FULL PRESCRIBING INFORMATION: CONTENTS*
- SHISEIDO WHITE LUCENT Uses
- Warnings
- Directions
- Inactive ingredients
- Questions?
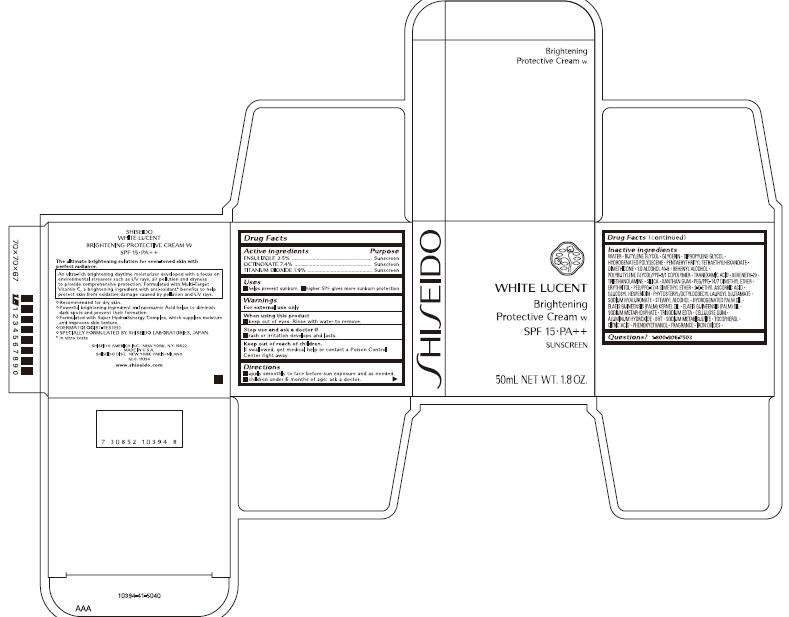
- PRINCIPAL DISPLAY PANEL - 50 mL Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active ingredients | Purpose |
| ENSULIZOLE 2.5% | Sunscreen |
| OCTINOXATE 7.4% | Sunscreen |
| TITANIUM DIOXIDE 1.9% | Sunscreen |
SHISEIDO WHITE LUCENT Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
Warnings
For external use only
When using this product
- keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- rash or irritation develops and lasts.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply smoothly to face before sun exposure and as needed.
- children under 6 months of age: ask a doctor.
Inactive ingredients
WATER, BUTYLENE GLYCOL, GLYCERIN, DIPROPYLENE GLYCOL, HYDROGENATED POLYDECENE, PENTAERYTHRITYL TETRAETHYLHEXANOATE, DIMETHICONE, SD ALCOHOL 40-B, BEHENYL ALCOHOL, POLYBUTYLENE GLYCOL/PPG-9/1 COPOLYMER, TRANEXAMIC ACID, BEHENETH-20, TRIETHANOLAMINE, SILICA, XANTHAN GUM, PEG/PPG-14/7 DIMETHYL ETHER, ERYTHRITOL, PEG/PPG-17/4 DIMETHYL ETHER, 2-O-ETHYL ASCORBIC ACID, GLUCOSYL HESPERIDIN, PHYTOSTERYL/OCTYLDODECYL LAUROYL GLUTAMATE, SODIUM HYALURONATE, STEARYL ALCOHOL, HYDROGENATED PALM OIL, ELAEIS GUINEENSIS (PALM) KERNEL OIL, ELAEIS GUINEENSIS (PALM) OIL, SODIUM METAPHOSPHATE, TRISODIUM EDTA, CELLULOSE GUM, ALUMINUM HYDROXIDE, BHT, SODIUM METABISULFITE, TOCOPHEROL, CITRIC ACID, PHENOXYETHANOL, FRAGRANCE, IRON OXIDES
Questions?
1-800-906-7503
SHISEIDO AMERICA INC. NEW YORK, N.Y. 10022
MADE IN U.S.A.
SHISEIDO DIST. NEW YORK • PARIS • MILANO
GLO. 10394
www.shiseido.com
PRINCIPAL DISPLAY PANEL - 50 mL Carton
SHISEIDO
WHITE LUCENT
Brightening
Protective Cream w
SPF 15 • PA++
SUNSCREEN
50mL NET WT. 1.8 OZ.
SHISEIDO WHITE LUCENTEnsulizole, Octinoxate, and Titanium Dioxide CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||