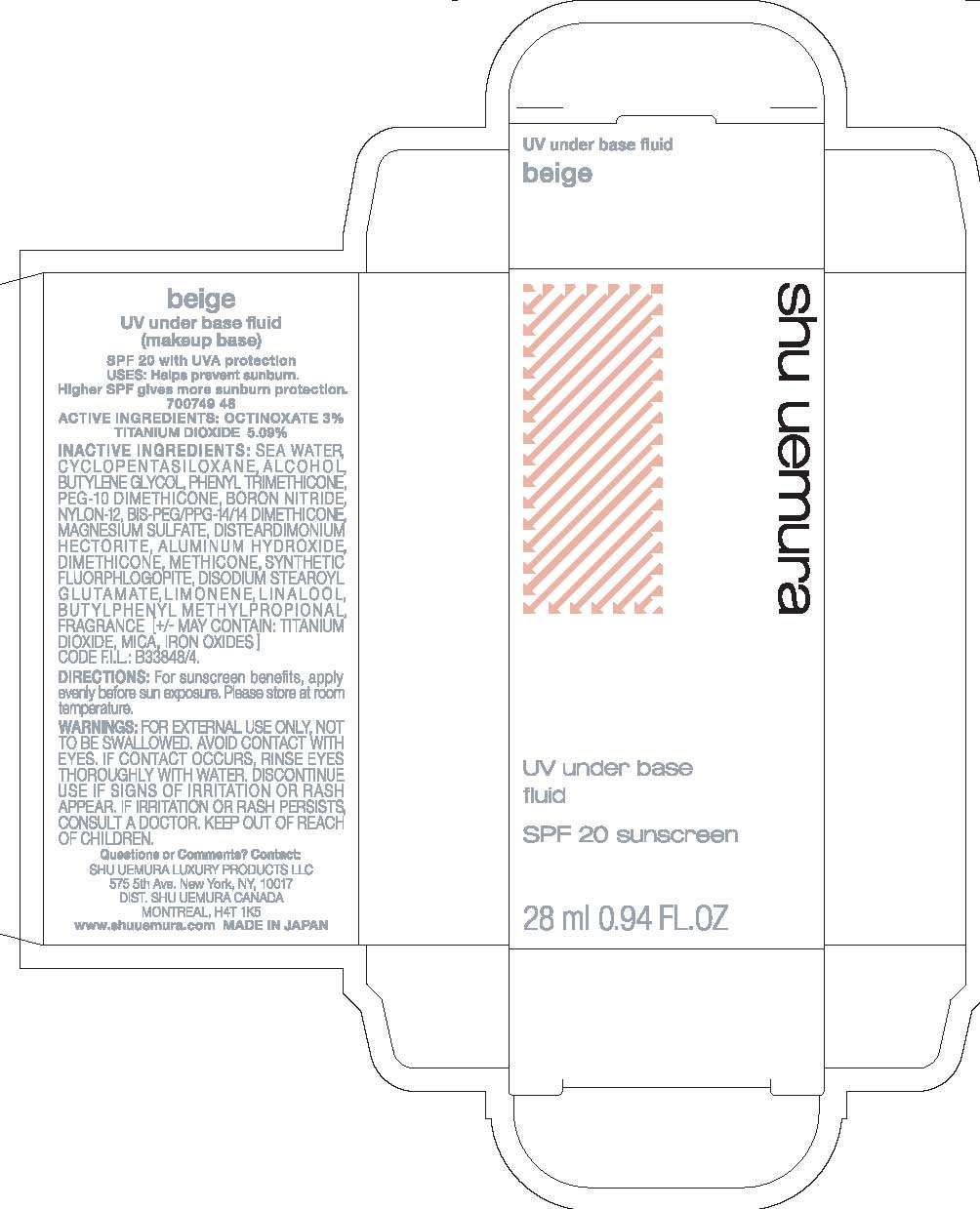
shu uemura UV underbase
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active Ingredients
OCTINOXATE 3%; TITANIUM DIOXIDE 5.09%
Uses: Directions:
USES: Helps prevent sunburn.
DIRECTIONS: For sunscreen benefits, apply evenly before sun exposure.
Storage:
Please store at room temperature.
Warnings
WARNINGS: FOR EXTERNAL USE ONLY, NOT TO BE SWALLOWED. AVOID CONTACT WITH EYES. IF CONTACT OCCURS, RINSE EYES THOROUGHLY WITH WATER. DISCONTINUE USE IF SIGNS OF IRRITATION OR RASH APPEAR. IF IRRITATION OR RASH PERSISTS, CONSULT A DOCTOR. KEEP OUT OF REACH OF CHILDREN.
shu uemura UV underbaseOctinoxate Titanium Dioxide LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!