Simvastatin
FULL PRESCRIBING INFORMATION: CONTENTS*
- SIMVASTATIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- CLINICAL STUDIES IN ADULTS
- INDICATIONS & USAGE
- SIMVASTATIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- SIMVASTATIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
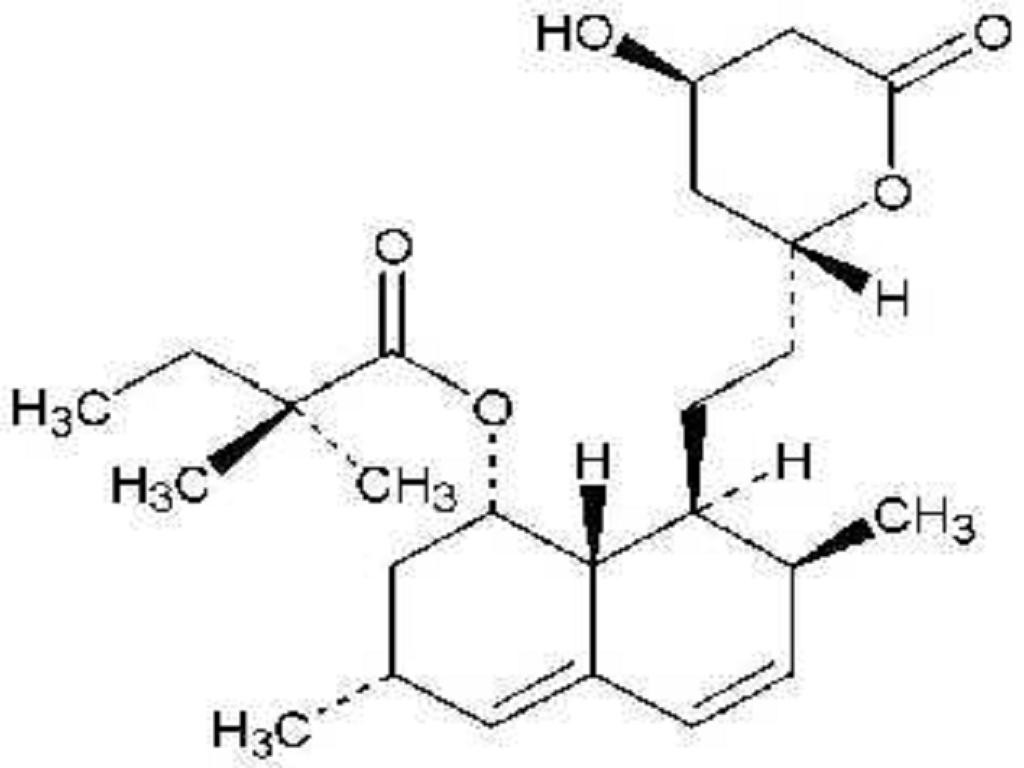
SIMVASTATIN DESCRIPTION
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
PRECAUTIONS, Geriatric Use
WARNINGS, Myopathy/RhabdomyolysisPRECAUTIONS, Drug Interactions
WARNINGS, Myopathy/RhabdomyolysisPRECAUTIONS, Drug InteractionsDOSAGE AND ADMINISTRATION
WARNINGS, Myopathy/RhabdomyolysisPRECAUTIONS, Drug Interactions
PRECAUTIONS, Drug Interactions11
1
CLINICAL STUDIES IN ADULTS
Reductions in Risk of CHD Mortality and Cardiovascular Events2
The HPS results showed that simvastatin 40 mg/day significantly reduced: total and CHD mortality; non-fatal myocardial infarctions, stroke, and revascularization procedures (coronary and non-coronary) (see Table 1).
TABLE 1: Summary of Heart Protection Study Results
EndpointSimvastatin(N = 10,269)n (%)Placebo (N = 10,267)n (%)Risk Reduction (%) (95% CI)p-Valuen = number of patients with indicated eventPrimaryMortality1,328 (12.9)1,507 (14.7)13(6 to 19)p = 0.0003CHD mortality587 (5.7)707 (6.9)18(8 to 26)p = 0.0005SecondaryNon-fatal MI357 (3.5)574 (5.6)38(30 to 46)p <0.0001Stroke444 (4.3)585 (5.7)25(15 to 34)p <0.0001TertiaryCoronary revascularization513 (5)725 (7.1)30(22 to 38)p <0.0001Peripheral and other non-coronary revascularization450 (4.4)532 (5.2)16(5 to 26)p = 0.006
Figure 1: The Effects of Treatment with Simvastatin on Major Vascular Events and Major Coronary Events in HPS

N = number of patients in each subgroup. The inverted triangles are point estimates of the relative risk, with their 95% confidence intervals represented as a line. The area of a triangle is proportional to the number of patients with MVE or MCE in the subgroup relative to the number with MVE or MCE, respectively, in the entire study population. The vertical solid line represents a relative risk of one. The vertical dashed line represents the point estimate of relative risk in the entire study population.
2
D.R. Taves, Minimization: a new method of assigning patients to treatment and control groups. Clin. Pharmacol. Ther. 15 (1974), pp. 443-453
Angiographic Studies
In the Multicenter Anti-Atheroma Study, the effect of simvastatin on atherosclerosis was assessed by quantitative coronary angiography in hypercholesterolemic patients with coronary heart disease. In this randomized, double-blind, controlled study, patients were treated with simvastatin 20 mg/day or placebo. Angiograms were evaluated at baseline, two and four years. The co-primary study endpoints were mean change per-patient in minimum and mean lumen diameters, indicating focal and diffuse disease, respectively. Simvastatin significantly slowed the progression of lesions as measured in the Year 4 angiogram by both parameters, as well as by change in percent diameter stenosis. In addition, simvastatin significantly decreased the proportion of patients with new lesions and with new total occlusions.
Modifications of Lipid Profiles
Primary Hypercholesterolemia (Fredrickson type lla and llb)
TABLE 2: Mean Response in Patients with Primary Hypercholesterolemia and Combined (mixed) Hyperlipidemia (Mean Percent Change from Baseline After 6 to 24 Weeks)
TREATMENTNTOTAL-CLDL-CHDL-CTG* *median percent changemean baseline LDL-C 244 mg/dL and median baseline TG 168 mg/dLmean baseline LDL-C 188 mg/dL and median baseline TG 128 mg/dLmean baseline LDL-C 226 mg/dL and median baseline TG 156 mg/dLmean baseline LDL-C 156 mg/dL and median baseline TG 391 mg/dLLower Dose Comparative Study(Mean % Change at Week 6)Simvastatin5 mg q.p.m.109-19-2610-12Simvastatin10 mg q.p.m.110-23-3012-15Scandinavian Simvastatin Survival Study(Mean % Change at Week 6)Placebo2223-1-10-2Simvastatin20 mg q.p.m.2221-28-388-19Upper Dose Comparative Study(Mean % Change Averaged atWeeks 18 and 24)Simvastatin 40 mg q.p.m.433-31-419-18Simvastatin 80 mg q.p.m.664-36-478-24Multi-Center Combined Hyperlipidemia Study(Mean % Change at Week 6)Placebo125123-4Simvastatin 40 mg q.p.m.123-25-2913-28Simvastatin80 mg q.p.m.124-31-3616-33In the Upper Dose Comparative Study, the mean reduction in LDL-C was 47% at the 80 mg dose. Of the 664 patients randomized to 80 mg, 475 patients with plasma TG200 mg/dL had a median reduction in TG of 21%, while in 189 patients with TG > 200 mg/dL, the median reduction in TG was 36%. In these studies, patients with TG > 350 mg/dL were excluded.
Hypertriglyceridemia (Fredrickson type lV)
The results of a subgroup analysis in 74 patients with type lV hyperlipidemia from a 130-patient, double-blind, placebo-controlled, 3-period crossover study are presented in Table 3.
TABLE 3: Six-week, Lipid-lowering Effects of Simvastatin in Type lV Hyperlipidemia Median Percent Change (25th and 75th percentile) from Baseline*
TREATMENTNTotal-CLDL-CHDL-CTGVLDL-CNon-HDL-C*The median baseline values (mg/dL) for the patients in this study were: total-C = 254, LDL-C = 135, HDL-C = 36, TG = 404, VLDL-C = 83, and non-HDL-C = 215.Placebo74+2(-7, +7)+1(-8, +14)+3(-3, +10)-9(-25, +13)-7(-25, +11)+1(-9, +8)Simvastatin40 mg/day74-25(-34, -19)-28(-40, -17)+11(+5, +23)-29(-43, -16)-37(-54, -23)-32(-42, -23)80 mg/day74-32(-38, -24)-37(-46, -26)+15(+5, +23)-34(-45, -18)-41(-57, -28)-38(-49, -32)
Dysbetalipoproteinemia (Fredrickson type lll)
The results of a subgroup analysis in 7 patients with type lll hyperlipidemia (dysbetalipoproteinemia) (apo E2/2) (VLDL-C/TG > 0.25) from a 130-patient, double-blind, placebo-controlled, 3-period crossover study are presented in Table 4. In this study the median baseline values (mg/dL) were: total-C = 324, LDL-C = 121, HDL-C = 31, TG = 411, VLDL-C = 170, and non HDL-C = 291.
Table 4: Six-week, Lipid-lowering Effects of Simvastatin in Type lll Hyperlipidemia Median Percent Change (min,max) from Baseline
TREATMENTNTotal-CLDL-C + IDLHDL-CTGVLDL-C+IDLNon-HDL-CPlacebo7-8(-24, +34)-8(-27, +23)-2(-21, +16)+4(-22, +90)-4(-28, +78)-8(-26, -39)Simvastatin 40 mg/day7-50(-66, -39)-50(-60, -31)+7(-8, +23)-41(-74, -16)-58(-90, -37)-57(-72, -44)Simvastatin 80 mg/day7-52(-55, -41)-51(-57, -28)+7(-5, +29)-38(-58, +2)-60(-72, -39)-59(-61, -46)
Homozygous Familial Hypercholesterolemia
In a controlled clinical study, 12 patients 15 to 39 years of age with homozygous familial hypercholesterolemia received simvastatin 40 mg/day in a single dose or in 3 divided doses, or 80 mg/day in 3 divided doses. Eleven of the 12 patients had reductions in LDL-C. In those patients with reductions, the mean LDL-C changes for the 40 and 80 mg doses were 14% (range 8% to 23%, median 12%) and 30% (range 14% to 46%, median 29%), respectively. One patient had an increase of 15% in LDL-C. Another patient with absent LDL-C receptor function had an LDL-C reduction of 41% with the 80 mg dose.
Endocrine Function
In clinical studies, simvastatin did not impair adrenal reserve or significantly reduce basal plasma cortisol concentration. Small reductions from baseline in basal plasma testosterone in men were observed in clinical studies with simvastatin, an effect also observed with other inhibitors of HMG-CoA reductase and the bile acid sequestrant cholestyramine. There was no effect on plasma gonadotropin levels. In a placebo-controlled, 12-week study there was no significant effect of simvastatin 80 mg on the plasma testosterone response to human chorionic gonadotropin (hCG). In another 24-week study, simvastatin 20 to 40 mg had no detectable effect on spermatogenesis. In 4S, in which 4,444 patients were randomized to simvastatin 20 to 40 mg/day or placebo for a median duration of 5.4 years, the incidence of male sexual adverse events in the two treatment groups was not significantly different. Because of these factors, the small changes in plasma testosterone are unlikely to be clinically significant. The effects, if any, on the pituitary-gonadal axis in pre-menopausal women are unknown.
Clinical Studies in Adolescents
In a double-blind, placebo-controlled study, 175 patients (99 adolescent boys and 76 post-menarchal girls) 10 to 17 years of age (mean age 14.1 years) with heterozygous familial hypercholesterolemia (heFH) were randomized to simvastatin (n = 106) or placebo (n = 67) for 24 weeks (base study). Inclusion in the study required a baseline LDL-C level between 160 and 400 mg/dL and at least one parent with an LDL-C level > 189 mg/dL. The dosage of simvastatin (once daily in the evening) was 10 mg for the first 8 weeks, 20 mg for the second 8 weeks, and 40 mg thereafter. In a 24-week extension, 144 patients elected to continue therapy and received simvastatin 40 mg or placebo.
Simvastatin significantly decreased plasma levels of total-C, LDL-C, and Apo B (see Table 5). Results from the extension at 48 weeks were comparable to those observed in the base study.
TABLE 5: Lipid-lowering Effects of Simvastatin in Adolescent Patients with Heterozygous Familial Hypercholesterolemia (Mean Percent Change from Baseline)
DosageDurationNTotal-CLDL-CHDL-CTG*Apo B*median percent changePlacebo24 Weeks67% Change from Baseline(95% CI)1.6(-2.2, 5.3)1.1(-3.4, 5.5)3.6(-0.7, 8)-3.2(-11.8, 5.4)-0.5(-4.7, 3.6)Mean baseline, mg/dL (SD)278.6(51.8)211.9(49)46.9(11.9)90(50.7)186.3(38.1)Simvastatin24 Weeks106% Change from Baseline (95% CI)-26.5(-29.6, -23.3)-36.8(-40.5, -33)8.3(4.6, 1.9)-7.9(-15.8, 0)-32.4 (-35.9, -29)Mean baseline, mg/dL (SD)270.2(44)203.8(41.5)47.7(9)78.3(46)179.9(33.8)After 24 weeks of treatment, the mean achieved LDL-C value was 124.9 mg/dL (range: 64to 289 mg/dL) in the simvastatin 40 mg group compared to 207.8 mg/dL (range: 128 to 334mg/dL) in the placebo group.
The safety and efficacy of doses above 40 mg daily have not been studied in children with heterozygous familial hypercholesterolemia. The long-term efficacy of simvastatin therapy in childhood to reduce morbidity and mortality in adulthood has not been established.
INDICATIONS & USAGE
National Cholesterol Education Program [NCEP] Treatment GuidelinesReductions in Risk of CHD Mortality and Cardiovascular Events
-
● Reduce the risk of total mortality by reducing CHD deaths.
-
● Reduce the risk of non-fatal myocardial infarction and stroke.
-
● Reduce the need for coronary and non-coronary revascularization procedures.
Patients with Hypercholesterolemia Requiring Modifications of Lipid Profiles
-
● Reduce elevated total-C, LDL-C, Apo B, and TG, and to increase HDL-C in patients with primary hypercholesterolemia (heterozygous familial and nonfamilial) and mixed dyslipidemia (Fredrickson types IIa and IIb).
-
● Treat patients with hypertriglyceridemia (Fredrickson type lV hyperlipidemia).
-
● Treat patients with primary dysbetalipoproteinemia (Fredrickson type lll hyperlipidemia).
-
● Reduce total-C and LDL-C in patients with homozygous familial hypercholesterolemia as an adjunct to other lipid-lowering treatments (e.g., LDL apheresis) or if such treatments are unavailable.
Adolescent Patients with Heterozygous Familial Hypercholesterolemia (HeFH)
-
● There is a positive family history of premature cardiovascular disease (CVD) or
-
● Two or more other CVD risk factors are present in the adolescent patient.
General Recommendations
*CHD*orCHD risk equivalents (10-year risk > 20%)< 100100130 (100 to 129: drug optional)S2+ Risk factors (10-year risk20%)< 13013010-year risk 10 to 20%:130 10-year risk <10%:1600 to 1 Risk factor< 160160190 (160 to 189: LDL-lowering drug optional)After the LDL-C goal has been achieved, if the TG is still200 mg/dL, non-HDL-C (total-C minus HDL-C) becomes a secondary target of therapy. Non-HDL-C goals are set 30 mg/dL higher than LDL-C goals for each risk category.
At the time of hospitalization for an acute coronary event, consideration can be given to initiating drug therapy at discharge.
The NCEP classification of cholesterol levels in pediatric patients with a familial history of either hypercholesterolemia or premature cardiovascular disease is summarized in Table 7.
TABLE 7: NCEP Classification of Cholesterol Levels in Pediatric Patients with a Familial History of Either HeFH or Premature CVD
CategoryTotal-C (mg/dL)LDL-C (mg/dL)Acceptable<170< 110Borderline170 to 199110 to 129High200130Since the goal of treatment is to lower LDL-C, the NCEP recommends that LDL-C levels be used to initiate and assess treatment response. Only if LDL-C levels are not available, should the total-C be used to monitor therapy.
Simvastatin is indicated to reduce elevated LDL-C and TG levels in patients with Type IIb hyperlipidemia (where hypercholesterolemia is the major abnormality). However, it has not been studied in conditions where the major abnormality is elevation of chylomicrons (i.e., hyperlipidemia Fredrickson types I and V)3.
SIMVASTATIN CONTRAINDICATIONS
WARNINGS
PRECAUTIONS, Pregnancy
WARNINGS
Myopathy/RhabdomyolysisCLINICAL PHARMACOLOGY, PharmacokineticsPRECAUTIONS, Drug InteractionsDOSAGE AND ADMINISTRATION).
TABLE 8: Drug Interactions Associated with Increased Risk of Myopathy/Rhabdomyolysis
Interacting AgentsPrescribing RecommendationsItraconazoleAvoid simvastatinKetoconazoleErythromycinClarithromycinTelithromycinHIV protease inhibitorsNefazodoneGemfibrozilDo not exceed 10 mg simvastatin dailyCyclosporineDanazolAmiodaroneDo not exceed 20 mg simvastatin dailyVerapamilGrapefruit juiceAvoid large quantities of grapefruit juice (> 1 quart daily)
Liver Dysfunction
Persistent increases (to more than 3X the ULN) in serum transaminases have occurred in approximately 1% of patients who received simvastatin in clinical studies. When drug treatment was interrupted or discontinued in these patients, the transaminase levels usually fell slowly to pretreatment levels. The increases were not associated with jaundice or other clinical signs or symptoms. There was no evidence of hypersensitivity.
In 4S (seeCLINICAL PHARMACOLOGY, Clinical Studies
In 2 controlled clinical studies in 1,105 patients, the 12 month incidence of persistent hepatic transaminase elevation without regard to drug relationship was 0.9% and 2.1% at the 40 and 80 mg dose, respectively. No patients developed persistent liver function abnormalities following the initial 6 months of treatment at a given dose.
It is recommended that liver function tests be performed before the initiation of treatment, and thereafter when clinically indicated. Patients titrated to the 80 mg dose should receive an additional test prior to titration, 3 months after titration to the 80 mg dose, and periodically thereafter (e.g., semiannually) for the first year of treatment. Patients who develop increased transaminase levels should be monitored with a second liver function evaluation to confirm the finding and be followed thereafter with frequent liver function tests until the abnormality(ies) return to normal. Should an increase in AST or ALT of 3X ULN or greater persist, withdrawal of therapy with simvastatin is recommended.
The drug should be used with caution in patients who consume substantial quantities of alcohol and/or have a past history of liver disease. Active liver diseases or unexplained transaminase elevations are contraindications to the use of simvastatin.
As with other lipid-lowering agents, moderate (less than 3X ULN) elevations of serum transaminases have been reported following therapy with simvastatin. These changes appeared soon after initiation of therapy with simvastatin, were often transient, were not accompanied by any symptoms and did not require interruption of treatment.
PRECAUTIONS
WARNINGSADVERSE REACTIONSINFORMATION FOR PATIENTS
Patients should be advised about substances they should not take concomitantly with simvastatin and be advised to report promptly unexplained muscle pain, tenderness, or weakness (see list below andWARNINGS, Myopathy/Rhabdomyolysis). Patients should also be advised to inform other physicians prescribing a new medication that they are taking simvastatin.DRUG INTERACTIONS
CYP3A4 InteractionsWARNINGS, Myopathy/RhabdomyolysisCLINICAL PHARMACOLOGY, Pharmacokinetics
Interactions with lipid-lowering drugs that can cause myopathy when given alone
WARNINGS, Myopathy/Rhabdomyolysis
DOSAGE AND ADMINISTRATION
Other drug interactions
CLINICAL PHARMACOLOGY,PharmacokineticsWARNINGS, Myopathy/Rhabdomyolysis).
Amiodarone or Verapamil: The risk of myopathy/rhabdomyolysis is increased by concomitant administration of amiodarone or verapamil with higher doses of simvastatin (seeWARNINGS, Myopathy/Rhabdomyolysis).
Propranolol: In healthy male volunteers there was a significant decrease in mean Cmax, but no change in AUC, for simvastatin total and active inhibitors with concomitant administration of single doses of simvastatin and propranolol. The clinical relevance of this finding is unclear. The pharmacokinetics of the enantiomers of propranolol were not affected.
Digoxin: Concomitant administration of a single dose of digoxin in healthy male volunteers receiving simvastatin resulted in a slight elevation (less than 0.3 ng/mL) in digoxin concentrations in plasma (as measured by a radioimmunoassay) compared to concomitant administration of placebo and digoxin. Patients taking digoxin should be monitored appropriately when simvastatin is initiated.
Warfarin: In two clinical studies, one in normal volunteers and the other in hypercholesterolemic patients, simvastatin 20, 40 mg/day modestly potentiated the effect of coumarin anticoagulants: the prothrombin time, reported as International Normalized Ratio (INR), increased from a baseline of 1.7 to 1.8 and from 2.6 to 3.4 in the volunteer and patient studies, respectively. With other reductase inhibitors, clinically evident bleeding and/or increased prothrombin time has been reported in a few patients taking coumarin anticoagulants concomitantly. In such patients, prothrombin time should be determined before starting simvastatin and frequently enough during early therapy to ensure that no significant alteration of prothrombin time occurs. Once a stable prothrombin time has been documented, prothrombin times can be monitored at the intervals usually recommended for patients on coumarin anticoagulants. If the dose of simvastatin is changed or discontinued, the same procedure should be repeated. Simvastatin therapy has not been associated with bleeding or with changes in prothrombin time in patients not taking anticoagulants.
CNS Toxicity
Optic nerve degeneration was seen in clinically normal dogs treated with simvastatin for 14 weeks at 180 mg/kg/day, a dose that produced mean plasma drug levels about 12 times higher than the mean plasma drug level in humans taking 80 mg/day.
A chemically similar drug in this class also produced optic nerve degeneration (Wallerian degeneration of retinogeniculate fibers) in clinically normal dogs in a dose-dependent fashion starting at 60 mg/kg/day, a dose that produced mean plasma drug levels about 30 times higher than the mean plasma drug level in humans taking the highest recommended dose (as measured by total enzyme inhibitory activity). This same drug also produced vestibulocochlear Wallerian-like degeneration and retinal ganglion cell chromatolysis in dogs treated for 14 weeks at 180 mg/kg/day, a dose that resulted in a mean plasma drug level similar to that seen with the 60 mg/kg/day dose.
CNS vascular lesions, characterized by perivascular hemorrhage and edema, mononuclear cell infiltration of perivascular spaces, perivascular fibrin deposits and necrosis of small vessels were seen in dogs treated with simvastatin at a dose of 360 mg/kg/day, a dose that produced mean plasma drug levels that were about 14 times higher than the mean plasma drug levels in humans taking 80 mg/day. Similar CNS vascular lesions have been observed with several other drugs of this class.
There were cataracts in female rats after two years of treatment with 50 and 100 mg/kg/day (22 and 25 times the human AUC at 80 mg/day, respectively) and in dogs after three months at 90 mg/kg/day (19 times) and at two years at 50 mg/kg/day (5 times).
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
CONTRAINDICATIONS
3CONTRAINDICATIONS
3
NURSING MOTHERS
CONTRAINDICATIONSPEDIATRIC USE
CLINICAL PHARMACOLOGY, Clinical Studies in AdolescentsADVERSE REACTIONS, Adolescent PatientsDOSAGE AND ADMINISTRATION, Adolescents (10 to 17 years of age) with Heterozygous Familial Hypercholesterolemia. Adolescent females should be counseled on appropriate contraceptive methods while on simvastatin therapy (seeCONTRAINDICATIONSandPRECAUTIONS, Pregnancy). Simvastatin has not been studied in patients younger than 10 years of age, nor in pre-menarchal girls.GERIATRIC USE
CLINICAL PHARMACOLOGYSIMVASTATIN ADVERSE REACTIONS
Clinical Adverse Experiences
In Adults
Scandinavian Simvastatin Survival Study
Clinical Adverse Experiences
CLINICAL PHARMACOLOGY, Clinical Studies
Heart Protection Study
Clinical Adverse Experiences
CLINICAL PHARMACOLOGY, Clinical Studies
Laboratory Tests
WARNINGS, Myopathy/Rhabdomyolysis
Concomitant Lipid-Lowering Therapy
WARNINGS, Myopathy/Rhabdomyolysis
Adolescent Patients (ages 10 to 17 years)
CLINICAL PHARMACOLOGY, Clinical Studies in AdolescentsPRECAUTIONS, Pediatric Use
OVERDOSAGE
DOSAGE & ADMINISTRATION
NCEP Treatment GuidelinesCLINICAL PHARMACOLOGY, Clinical Studies in AdultsPatients with Homozygous Familial Hypercholesterolemia
Adolescents (10 to 17 years of age) with Heterozygous Familial Hypercholesterolemia
4CLINICAL PHARMACOLOGY
4
Concomitant LipidLowering Therapy
WARNINGS, Myopathy/RhabdomyolysisPRECAUTIONS, Drug Interactions
Patients taking Cyclosporine or Danazol
WARNINGS, Myopathy/Rhabdomyolysis
Patients taking Amiodarone or Verapamil
WARNINGS, Myopathy/RhabdomyolysisPRECAUTIONS, Drug Interactions, Other drug interactions
Patients with Renal Insufficiency
CLINICAL PHARMACOLOGY, PharmacokineticsWARNINGS, Myopathy/Rhabdomyolysis
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

SimvastatinSimvastatin TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!