SIVEXTRO
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SIVEXTRO™ safely and effectively. See full prescribing information for SIVEXTRO. SIVEXTRO (tedizolid phosphate) for injection, for intravenous use SIVEXTRO (tedizolid phosphate) tablet, for oral use Initial U.S. Approval: 2014INDICATIONS AND USAGESIVEXTRO is an oxazolidinone-class antibacterial drug indicated in adults for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by designated susceptible bacteria. (1)To reduce the development of drug-resistant bacteria and maintain the effectiveness of SIVEXTRO and other antibacterial drugs, SIVEXTRO should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. DOSAGE AND ADMINISTRATION200 mg administered once daily orally or as an intravenous (IV) infusion over 1 hour for six (6) days. (2.1)DOSAGE FORMS AND STRENGTHS For injection: 200 mg, sterile, lyophilized powder in single-use vial for reconstitution for intravenous infusion; Tablet: 200 mg (3) CONTRAINDICATIONSNone (4)WARNINGS AND PRECAUTIONS Patients with neutropenia: The safety and efficacy of SIVEXTRO in patients with neutropenia (neutrophil counts
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 SIVEXTRO INDICATIONS AND USAGE
- 2 SIVEXTRO DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 SIVEXTRO CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 SIVEXTRO ADVERSE REACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 SIVEXTRO DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PRINCIPAL DISPLAY PANEL - 200 mg Tablet Blister Pack Carton
- PRINCIPAL DISPLAY PANEL - 200 mg Vial Carton
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Acute Bacterial Skin and Skin Structure Infections
SIVEXTRO™ is an oxazolidinone-class antibacterial indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by susceptible isolates of the following Gram-positive microorganisms: Staphylococcus aureus (including methicillin-resistant [MRSA] and methicillin-susceptible [MSSA] isolates), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus anginosus Group (including Streptococcus anginosus, Streptococcus intermedius, and Streptococcus constellatus), and Enterococcus faecalis.
1.2 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of SIVEXTRO and other antibacterial drugs, SIVEXTRO should be used only to treat ABSSSI that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of SIVEXTRO is 200 mg administered once daily for six (6) days either orally (with or without food) or as an intravenous (IV) infusion in patients 18 years of age or older.
The recommended dosage and administration is described in Table 1.
| Infection | Route | Dosage | Frequency | Infusion Time | Duration of Treatment |
|---|---|---|---|---|---|
| Acute Bacterial Skin and Skin Structure Infection (ABSSSI) | Intravenous | 200 mg | Once daily | 1 hour | 6 days |
| Oral | 200 mg | Once daily | Not Applicable |
No dose adjustment is necessary when changing from intravenous to oral SIVEXTRO.
If patients miss a dose, they should take it as soon as possible anytime up to 8 hours prior to their next scheduled dose. If less than 8 hours remain before the next dose, wait until their next scheduled dose.
2.2 Preparation and Administration of Intravenous Solution
SIVEXTRO is supplied as a sterile, lyophilized powder for injection in single-use vials of 200 mg. Each 200 mg vial must be reconstituted with Sterile Water for Injection and subsequently diluted only with 0.9% Sodium Chloride Injection, USP.
SIVEXTRO vials contain no antimicrobial preservatives and are intended for single use only.
Preparation
The contents of the vial should be reconstituted using aseptic technique as follows:
Note: To minimize foaming, AVOID vigorous agitation or shaking of the vial during or after reconstitution.
- Reconstitute the SIVEXTRO vial with 4 mL of Sterile Water for Injection.
- Gently swirl the contents and let the vial stand until the cake has completely dissolved and any foam disperses.
- Inspect the vial to ensure the solution contains no particulate matter and no cake or powder remains attached to the sides of the vial. If necessary, invert the vial to dissolve any remaining powder and swirl gently to prevent foaming. The reconstituted solution is clear and colorless to pale-yellow in color; the total storage time should not exceed 24 hours at either room temperature or under refrigeration at 2°C to 8°C (36°F to 46°F).
- Tilt the upright vial and insert a syringe with appropriately sized needle into the bottom corner of the vial and remove 4 mL of the reconstituted solution. Do not invert the vial during extraction.
- The reconstituted solution must be further diluted in 250 mL of 0.9% Sodium Chloride Injection, USP. Slowly inject the 4 mL of reconstituted solution into a 250 mL bag of 0.9% Sodium Chloride Injection, USP. Invert the bag gently to mix. Do NOT shake the bag as this may cause foaming.
Administration
Administer as an intravenous infusion only.
Do not administer as an intravenous push or bolus. Do not mix SIVEXTRO with other drugs when administering. It is not intended for intra-arterial, intramuscular, intrathecal, intraperitoneal, or subcutaneous administration.
The intravenous bag containing the reconstituted and diluted intravenous solution should be inspected visually for particulate matter prior to administration. Discard if visible particles are observed. The resulting solution is clear and colorless to pale-yellow in color.
After reconstitution and dilution, SIVEXTRO is to be administered via intravenous infusion using a total time of 1 hour.
The total time from reconstitution to administration should not exceed 24 hours at room temperature or under refrigeration at 2°C to 8°C (36°F to 46°F).
2.3 Compatible Intravenous Solutions
SIVEXTRO is compatible with 0.9% Sodium Chloride Injection, USP.
2.4 Incompatibilities
SIVEXTRO for injection is incompatible with any solution containing divalent cations (e.g., Ca2+, Mg2+), including Lactated Ringer's Injection and Hartmann's Solution.
Limited data are available on the compatibility of SIVEXTRO for injection with other intravenous substances, additives or other medications and they should not be added to SIVEXTRO single-use vials or infused simultaneously. If the same intravenous line is used for sequential infusion of several different drugs, the line should be flushed before and after infusion of SIVEXTRO with 0.9% Sodium Chloride Injection, USP.
3 DOSAGE FORMS AND STRENGTHS
SIVEXTRO 200 mg tablet is a yellow film-coated oval tablet; each tablet is debossed with "TZD" on one side and "200" on the other side.
SIVEXTRO for injection is a sterile, white to off-white lyophilized powder for injection in single-use vials of 200 mg. Each 200 mg vial must be reconstituted with Sterile Water for Injection and subsequently diluted only with 0.9% Sodium Chloride Injection, USP.
4 CONTRAINDICATIONS
None
5 WARNINGS AND PRECAUTIONS
5.1 Patients with Neutropenia
The safety and efficacy of SIVEXTRO in patients with neutropenia (neutrophil counts <1000 cells/mm3) have not been adequately evaluated. In an animal model of infection, the antibacterial activity of SIVEXTRO was reduced in the absence of granulocytes [see Clinical Pharmacology (12.2) ]. Alternative therapies should be considered when treating patients with neutropenia and acute bacterial skin and skin structure infection.
5.2 -Associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported for nearly all systemic antibacterial agents including SIVEXTRO, with severity ranging from mild diarrhea to fatal colitis. Treatment with antibacterial agents can alter the normal flora of the colon and may permit overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antibacterial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary because CDAD has been reported to occur more than two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, antibacterial use not directed against C. difficile should be discontinued, if possible. Appropriate measures such as fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.3 Development of Drug-Resistant Bacteria
Prescribing SIVEXTRO in the absence of a proven or strongly suspected bacterial infection or prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
6 ADVERSE REACTIONS
6.1 Side Effects in Clinical Trials
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be compared directly to rates from clinical trials of another drug and may not reflect rates observed in practice.
Adverse reactions were evaluated for 1050 patients treated with SIVEXTRO and 662 patients treated with the comparator antibacterial drug in two Phase 2 and two Phase 3 clinical trials. The median age of patients treated with SIVEXTRO in the Phase 2 and Phase 3 trials was 42 years, ranging between 17 and 86 years old. Patients treated with SIVEXTRO were predominantly male (65%) and White (82%).
Serious Adverse Reactions and Adverse Reactions Leading to Discontinuation
Serious adverse reactions occurred in 12/662 (1.8%) of patients treated with SIVEXTRO and in 13/662 (2.0%) of patients treated with the comparator. SIVEXTRO was discontinued due to an adverse reaction in 3/662 (0.5%) of patients and the comparator was discontinued due to an adverse reaction in 6/662 (0.9%) of patients.
Most Common Adverse Reactions
The most common adverse reactions in patients treated with SIVEXTRO were nausea (8%), headache (6%), diarrhea (4%), vomiting (3%), and dizziness (2%). The median time of onset of adverse reactions was 5 days for both SIVEXTRO and linezolid with 12% occurring on the second day of treatment in both treatment groups.
Table 2 lists selected adverse reactions occurring in at least 2% of patients treated with SIVEXTRO in clinical trials.
| Adverse Reactions | Pooled Phase 3 ABSSSI Clinical Trials | |
|---|---|---|
| SIVEXTRO (200 mg oral/intravenous once daily for 6 days) (N=662) |
Linezolid (600 mg oral/intravenous twice daily for 10 days) (N=662) |
|
| Gastrointestinal Disorders | ||
| Nausea | 8% | 12% |
| Diarrhea | 4% | 5% |
| Vomiting | 3% | 6% |
| Nervous System Disorder | ||
| Headache | 6% | 6% |
| Dizziness | 2% | 2% |
The following selected adverse reactions were reported in SIVEXTRO-treated patients at a rate of less than 2% in these clinical trials:
Blood and Lymphatic System Disorders: anemia
Cardiovascular: palpitations, tachycardia
Eye Disorders: asthenopia, vision blurred, visual impairment, vitreous floaters
General Disorders and Administration Site Conditions: infusion-related reactions
Immune System Disorders: drug hypersensitivity
Infections and Infestations: Clostridium difficile colitis, oral candidiasis, vulvovaginal mycotic infection
Investigations: hepatic transaminases increased, white blood cell count decreased
Nervous System Disorders: hypoesthesia, paresthesia, VIIth nerve paralysis
Psychiatric Disorders: insomnia
Skin and Subcutaneous Tissue Disorders: pruritus, urticaria, dermatitis
Vascular Disorders: flushing, hypertension
Laboratory Parameters
Hematology laboratory abnormalities that were determined to be potentially clinically significant in the pooled Phase 3 ABSSSI clinical trials are provided in Table 3.
| Laboratory Assay | Potentially Clinically Significant Values |
|
|---|---|---|
| SIVEXTRO (200 mg oral/intravenous once daily for 6 days) (N=618) |
Linezolid (600 mg oral/intravenous twice daily for 10 days) (N=617) |
|
| M = male; F = female | ||
| Hemoglobin (<10.1 g/dL [M]) (<9 g/dL [F]) |
3.1% | 3.7% |
| Platelet count (<112 × 103/mm3) |
2.3% | 4.9% |
| Absolute neutrophil count (<0.8 × 103/mm3) |
0.5% | 0.6% |
Myelosuppression
Phase 1 studies conducted in healthy adults exposed to SIVEXTRO for 21 days showed a possible dose and duration effect on hematologic parameters beyond 6 days of treatment. In the Phase 3 trials, clinically significant changes in these parameters were generally similar for both treatment arms (see Table 3).
Peripheral and Optic Neuropathy
Peripheral and optic neuropathy have been described in patients treated with another member of the oxazolidinone class for longer than 28 days. In Phase 3 trials, reported adverse reactions for peripheral neuropathy and optic nerve disorders were similar between both treatment arms (peripheral neuropathy 1.2% vs. 0.6% for tedizolid phosphate and linezolid, respectively; optic nerve disorders 0.3% vs. 0.2%, respectively). No data are available for patients exposed to SIVEXTRO for longer than 6 days.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies of SIVEXTRO in pregnant women. SIVEXTRO should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In embryo-fetal studies, tedizolid phosphate was shown to produce fetal developmental toxicities in mice, rats, and rabbits. Fetal developmental effects occurring in mice in the absence of maternal toxicity included reduced fetal weights and an increased incidence of costal cartilage anomalies at the high dose of 25 mg/kg/day (4-fold the estimated human exposure level based on AUCs). In rats, decreased fetal weights and increased skeletal variations including reduced ossification of the sternabrae, vertebrae, and skull were observed at the high dose of 15 mg/kg/day (6-fold the estimated human exposure based on AUCs) and were associated with maternal toxicity (reduced maternal body weights). In rabbits, reduced fetal weights but no malformations or variations were observed at doses associated with maternal toxicity. The no observed adverse effect levels (NOAELs) for fetal toxicity in mice (5 mg/kg/day), maternal and fetal toxicity in rats (2.5 mg/kg/day), and rabbits (1 mg/kg/day) were associated with tedizolid plasma area under the curve (AUC) values approximately equivalent to (mice and rats) or 0.04-fold (rabbit) the tedizolid AUC value associated with the oral human therapeutic dose.
In a pre-postnatal study, there were no adverse maternal or offspring effects when female rats were treated during pregnancy and lactation with tedizolid phosphate at the highest tested dose of 3.75 mg/kg/day, with plasma tedizolid exposure (AUC) approximately equivalent to the human plasma AUC exposure at the clinical dose of 200 mg/day.
8.3 Nursing Mothers
Tedizolid is excreted in the breast milk of rats. It is not known whether tedizolid is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when SIVEXTRO is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients below the age of 18 have not been established.
8.5 Geriatric Use
Clinical studies of SIVEXTRO did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. No overall differences in pharmacokinetics were observed between elderly subjects and younger subjects.
10 OVERDOSAGE
In the event of overdosage, SIVEXTRO should be discontinued and general supportive treatment given. Hemodialysis does not result in meaningful removal of tedizolid from systemic circulation.
11 DESCRIPTION
SIVEXTRO (tedizolid phosphate), a phosphate prodrug, is converted to tedizolid in the presence of phosphatases.
Tedizolid phosphate has the chemical name [(5R)-(3-{3-Fluoro-4-[6-(2-methyl-2H-tetrazol- 5-yl) pyridin-3-yl]phenyl}-2-oxooxazolidin- 5-yl]methyl hydrogen phosphate.
Its empirical formula is C17H16FN6O6P and its molecular weight is 450.32. Its structural formula is:
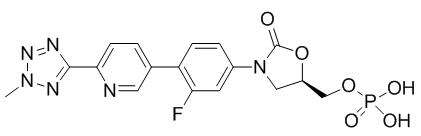
Tedizolid phosphate is a white to yellow solid and is administered orally or by intravenous infusion.
The pharmacologically active moiety, tedizolid, is an antibacterial agent of the oxazolidinone class.
SIVEXTRO tablets contain 200 mg of tedizolid phosphate, and the following inactive ingredients: microcrystalline cellulose, mannitol, crospovidone, povidone, and magnesium stearate. In addition, the film coating contains the following inactive ingredients: polyvinyl alcohol, titanium dioxide, polyethylene glycol/macrogol, talc, and yellow iron oxide.
SIVEXTRO for injection is a sterile, white to off-white sterile lyophilized powder for injection in single-use vials of 200 mg. The inactive ingredients are mannitol (105 mg), sodium hydroxide, and hydrochloric acid, which is used in minimal quantities for pH adjustment.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tedizolid phosphate is the prodrug of tedizolid, an antibacterial agent [see Pharmacokinetics (12.3); Microbiology (12.4) ].
12.2 Pharmacodynamics
The AUC/minimum inhibitory concentration (MIC) was shown to best correlate with tedizolid activity in animal infection models.
In the mouse thigh infection model of S. aureus, antistaphylococcal killing activity was impacted by the presence of granulocytes. In granulocytopenic mice (neutrophil count <100 cells/mL), bacterial stasis was achieved at a human-equivalent dose of approximately 2000 mg/day; whereas, in non-granulocytopenic animals, stasis was achieved at a human-equivalent dose of approximately 100 mg/day. The safety and efficacy of SIVEXTRO for the treatment of neutropenic patients (neutrophil counts <1000 cells/mm3) have not been evaluated.
Cardiac Electrophysiology
In a randomized, positive- and placebo-controlled crossover thorough QTc study, 48 enrolled subjects were administered a single oral dose of SIVEXTRO at a therapeutic dose of 200 mg, SIVEXTRO at a supratherapeutic dose of 1200 mg, placebo, and a positive control; no significant effects of SIVEXTRO on heart rate, electrocardiogram morphology, PR, QRS, or QT interval were detected. Therefore, SIVEXTRO does not affect cardiac repolarization.
12.3 Pharmacokinetics
Tedizolid phosphate is a prodrug that is converted by phosphatases to tedizolid, the microbiologically active moiety, following oral and intravenous administration. Only the pharmacokinetic profile of tedizolid is discussed further due to negligible systemic exposure of tedizolid phosphate following oral and intravenous administration. Following multiple once-daily oral or intravenous administration, steady-state concentrations are achieved within approximately three days with tedizolid accumulation of approximately 30% (tedizolid half-life of approximately 12 hours). Pharmacokinetic (PK) parameters of tedizolid following oral and intravenous administration of 200 mg once daily tedizolid phosphate are shown in Table 4.
| Pharmacokinetic Parameters of Tedizolid |
Oral | Intravenous | ||
|---|---|---|---|---|
| Single Dose | Steady State | Single Dose | Steady State | |
| Cmax (mcg/mL) | 2.0 (0.7) | 2.2 (0.6) | 2.3 (0.6) | 3.0 (0.7) |
| Tmax (hr) |
2.5 (1.0 - 8.0) | 3.5 (1.0 - 6.0) | 1.1 (0.9 - 1.5) | 1.2 (0.9 - 1.5) |
| AUC (mcg•hr/mL) |
23.8 (6.8) | 25.6 (8.4) | 26.6 (5.2) | 29.2 (6.2) |
| CL or CL/F (L/hr) | 6.9 (1.7) | 8.4 (2.1) | 6.4 (1.2) | 5.9 (1.4) |
Absorption
Peak plasma tedizolid concentrations are achieved within approximately 3 hours following oral administration under fasting conditions or at the end of the 1 hour intravenous infusion of tedizolid phosphate. The absolute bioavailability is approximately 91% and no dosage adjustment is necessary between intravenous and oral administration.
Tedizolid phosphate (oral) may be administered with or without food as total systemic exposure (AUC0- ∞) is unchanged between fasted and fed (high-fat, high-calorie) conditions.
Distribution
Protein binding of tedizolid to human plasma proteins is approximately 70 to 90%. The mean steady state volume of distribution of tedizolid in healthy adults following a single intravenous dose of tedizolid phosphate 200 mg ranged from 67 to 80 L (approximately twice total body water). Tedizolid penetrates into the interstitial space fluid of adipose and skeletal muscle tissue with exposure similar to free drug exposure in plasma.
Metabolism
Other than tedizolid, which accounts for approximately 95% of the total radiocarbon AUC in plasma, there are no other significant circulating metabolites in humans.
There was no degradation of tedizolid in human liver microsomes indicating tedizolid is unlikely to be a substrate for hepatic CYP450 enzymes.
Excretion
Following single oral administration of 14C-labeled tedizolid phosphate under fasted conditions, the majority of elimination occurred via the liver, with 82% of the radioactive dose recovered in feces and 18% in urine, primarily as a non-circulating and microbiologically inactive sulfate conjugate. Most of the elimination of tedizolid (>85%) occurs within 96 hours. Less than 3% of the tedizolid phosphate-administered dose is excreted in feces and urine as unchanged tedizolid.
Specific Populations
Based on the population pharmacokinetic analysis, there are no clinically relevant demographic or clinical patient factors (including age, gender, race, ethnicity, weight, body mass index, and measures of renal or liver function) that impact the pharmacokinetics of tedizolid.
Hepatic Impairment
Following administration of a single 200 mg oral dose of SIVEXTRO, no clinically meaningful changes in mean tedizolid Cmax and AUC0- ∞ were observed in patients with moderate (n=8) or severe (n=8) hepatic impairment (Child-Pugh Class B and C) compared to 8 matched healthy control subjects. No dose adjustment is necessary for patients with hepatic impairment.
Renal Impairment
Following administration of a single 200 mg intravenous dose of SIVEXTRO to 8 subjects with severe renal impairment defined as eGFR <30 mL/min/1.73 m2, the Cmax was essentially unchanged and AUC0- ∞ was decreased by less than 10% compared to 8 matched healthy control subjects. Hemodialysis does not result in meaningful removal of tedizolid from systemic circulation, as assessed in subjects with end-stage renal disease (eGFR <15 mL/min/1.73 m2). No dosage adjustment is necessary in patients with renal impairment or patients on hemodialysis.
Geriatric Patients
The pharmacokinetics of tedizolid were evaluated in a Phase 1 study conducted in elderly healthy volunteers (age 65 years and older, with at least 5 subjects at least 75 years old; n=14) compared to younger control subjects (25 to 45 years old; n=14) following administration of a single oral dose of SIVEXTRO 200 mg. There were no clinically meaningful differences in tedizolid Cmax and AUC0- ∞ between elderly subjects and younger control subjects. No dosage adjustment of SIVEXTRO is necessary in elderly patients.
Gender
The impact of gender on the pharmacokinetics of SIVEXTRO was evaluated in clinical trials of healthy males and females and in a population pharmacokinetics analysis. The pharmacokinetics of tedizolid were similar in males and females. No dosage adjustment of SIVEXTRO is necessary based on gender.
Drug Interaction Studies
Drug Metabolizing Enzymes
Transformation via Phase 1 hepatic oxidative metabolism is not a significant pathway for elimination of SIVEXTRO.
Neither SIVEXTRO nor tedizolid detectably inhibited or induced the metabolism of selected CYP enzyme substrates. No potential drug interactions with tedizolid were identified in in vitro CYP inhibition or induction studies. These results suggest that drug-drug interactions based on oxidative metabolism are unlikely.
Membrane Transporters
The potential for tedizolid or tedizolid phosphate to inhibit transport of probe substrates of important drug uptake (OAT1, OAT3, OATP1B1, OATP1B3, OCT1, and OCT2) and efflux transporters (P-gp and ABCG2 [also known as BCRP]) was tested in vitro. No clinically significant inhibition of any transporter was observed at tedizolid circulating plasma concentrations up to the Cmax.
Monoamine Oxidase Inhibition
Tedizolid is a reversible inhibitor of monoamine oxidase (MAO) in vitro. The interaction with MAO inhibitors could not be evaluated in Phase 2 and 3 trials, as subjects taking such medications were excluded from the trials.
Adrenergic Agents
Two placebo-controlled crossover studies were conducted to assess the potential of 200 mg oral SIVEXTRO at steady state to enhance pressor responses to pseudoephedrine and tyramine in healthy individuals. No meaningful changes in blood pressure or heart rate were seen with pseudoephedrine. The median tyramine dose required to cause an increase in systolic blood pressure of ≥30 mmHg from pre-dose baseline was 325 mg with SIVEXTRO compared to 425 mg with placebo. Palpitations were reported in 21/29 (72.4%) subjects exposed to SIVEXTRO compared to 13/28 (46.4%) exposed to placebo in the tyramine challenge study.
Serotonergic Agents
Serotonergic effects at doses of tedizolid phosphate up to 30-fold above the human equivalent dose did not differ from vehicle control in a mouse model that predicts serotonergic activity.
In Phase 3 trials, subjects taking serotonergic agents including antidepressants such as selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, and serotonin 5-hydroxytryptamine (5-HT1) receptor agonists (triptans), meperidine, or buspirone were excluded.
12.4 Microbiology
Tedizolid belongs to the oxazolidinone class of antibacterial drugs.
Mechanism of Action
The antibacterial activity of tedizolid is mediated by binding to the 50S subunit of the bacterial ribosome resulting in inhibition of protein synthesis. Tedizolid inhibits bacterial protein synthesis through a mechanism of action different from that of other non-oxazolidinone class antibacterial drugs; therefore, cross-resistance between tedizolid and other classes of antibacterial drugs is unlikely. The results of in vitro time-kill studies show that tedizolid is bacteriostatic against enterococci, staphylococci, and streptococci.
Mechanism of Resistance
Organisms resistant to oxazolidinones via mutations in chromosomal genes encoding 23S rRNA or ribosomal proteins (L3 and L4) are generally cross-resistant to tedizolid. In the limited number of Staphylococcus aureus strains tested, the presence of the chloramphenicol-florfenicol resistance (cfr) gene did not result in resistance to tedizolid in the absence of chromosomal mutations.
Frequency of Resistance
Spontaneous mutations conferring reduced susceptibility to tedizolid occur in vitro at a frequency rate of approximately 10-10.
Interaction with Other Antimicrobial Drugs
In vitro drug combination studies with tedizolid and aztreonam, ceftriaxone, ceftazidime, imipenem, rifampin, trimethoprim/sulfamethoxazole, minocycline, clindamycin, ciprofloxacin, daptomycin, vancomycin, gentamicin, amphotericin B, ketoconazole, and terbinafine demonstrate neither synergy nor antagonism.
Spectrum of Activity
Tedizolid has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections, as described in Indications and Usage (1).
Aerobic and Facultative Gram-positive Bacteria
-
-
-
-
-
The following in vitro data are available, but their clinical significance has not been established. At least 90% of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to 0.5 mcg/mL for tedizolid. However, the safety and effectiveness of SIVEXTRO in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
Aerobic and Facultative Anaerobic Gram-positive Bacteria
-
-
-
-
Susceptibility Test Methods
When available, the clinical microbiology laboratory should provide cumulative results of the in vitro susceptibility test results for antimicrobial drugs used in local hospitals and practice areas to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting an effective antibacterial drug for treatment.
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MIC values provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MIC values should be determined using a standardized procedure based on dilution methods (broth, agar, or microdilution) or equivalent using standardized inoculum and concentrations of tedizolid.1, 3 The MIC values should be interpreted according to the criteria provided in Table 5.
| Pathogen | Minimum Inhibitory Concentrations (mcg/mL) |
Disk Diffusion Zone Diameter (mm) |
||||
|---|---|---|---|---|---|---|
| S | I | R | S | I | R | |
| S=susceptible, I=intermediate, R=resistant | ||||||
|
Staphylococcus aureus (methicillin-resistant and methicillin-susceptible isolates) |
≤0.5 | 1 | ≥2 | ≥19 | 16 - 18 | ≤15 |
| Streptococcus pyogenes | ≤0.5 | - | - | ≥18 | - | - |
| Streptococcus agalactiae | ≤0.5 | - | - | ≥18 | - | - |
|
Streptococcus anginosus Group |
≤0.25 | - | - | ≥17 | - | - |
| Enterococcus faecalis | ≤0.5 | - | - | ≥19 | - | - |
Diffusion techniques
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The standardized procedure requires the use of standardized inoculum concentrations.2, 3 This procedure uses paper disks impregnated with 20 mcg tedizolid to test the susceptibility of microorganisms to tedizolid. Reports from the laboratory providing results of the standard single-disk susceptibility test with a 20 mcg tedizolid disk should be interpreted according to the criteria in Table 5.
A report of "Susceptible" indicates that the antimicrobial drug is likely to inhibit growth of the pathogen if the antimicrobial drug reaches the concentration usually achievable at the site of infection. A report of "Intermediate" indicates that the result should be considered equivocal, and if the microorganism is not fully susceptible to alternative drugs, the test should be repeated. This category implies possible clinical efficacy in body sites where the drug is physiologically concentrated. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the antimicrobial drug is not likely to inhibit growth of the pathogen if the antimicrobial drug reaches the concentrations usually achievable at the infection site; other therapy should be selected.
Quality Control
Standardized susceptibility test procedures require the use of laboratory control microorganisms to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individuals performing the test.1, 2, 3 Standardized tedizolid powder should provide the following range of MIC values noted in Table 6. For the diffusion technique using the 20 mcg tedizolid disk, results within the ranges specified in Table 6 should be observed.
| Quality Control Organism | Minimum Inhibitory Concentrations (mcg/mL) | Disk Diffusion (zone diameter in mm) |
|---|---|---|
|
Staphylococcus aureus
ATCC 29213 |
0.25 - 1 | Not Applicable |
|
Staphylococcus aureus
ATCC 25923 |
Not Applicable | 22 - 29 |
|
Enterococcus faecalis
ATCC 29212 |
0.25 - 1 | Not Applicable |
|
Streptococcus pneumoniae
ATCC 49619 |
0.12 - 0.5 | 24 - 30 |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies have not been conducted with tedizolid phosphate.
Tedizolid phosphate was negative for genotoxicity in all in vitro assays (bacterial reverse mutation (Ames), Chinese hamster lung (CHL) cell chromosomal aberration) and in all in vivo tests (mouse bone marrow micronucleus, rat liver unscheduled DNA synthesis). Tedizolid, generated from tedizolid phosphate after metabolic activation (in vitro and in vivo), was also tested for genotoxicity. Tedizolid was positive in an in vitro CHL cell chromosomal aberration assay, but negative for genotoxicity in other in vitro assays (Ames, mouse lymphoma mutagenicity) and in vivo in a mouse bone marrow micronucleus assay.
In a fertility study, oral tedizolid phosphate had no adverse effects on the fertility or reproductive performance, including spermatogenesis, of male rats at the maximum tested dose (50 mg/kg/day) with a plasma tedizolid AUC approximately 5-fold greater than the plasma AUC value in humans at the oral therapeutic dose. Tedizolid phosphate also had no adverse effects on the fertility or reproductive performance of adult female rats at doses up to the maximum tested (15 mg/kg/day). Plasma tedizolid exposure (AUC) at this NOAEL in female rats was approximately 4-fold higher than that in humans at the oral therapeutic dose.
13.2 Animal Toxicity and/or Pharmacology
Repeated-oral and intravenous dosing of tedizolid phosphate in rats in 1-month and 3-month toxicology studies produced dose- and time-dependent bone marrow hypocellularity (myeloid, erythroid, and megakaryocyte), with associated reduction in circulating RBCs, WBCs, and platelets. These effects showed evidence of reversibility and occurred at plasma tedizolid exposure levels (AUC) ≥6-fold greater than the plasma exposure associated with the human therapeutic dose. In a 1-month immunotoxicology study in rats, repeated oral dosing of tedizolid phosphate was shown to significantly reduce splenic B cells and T cells and reduce plasma IgG titers. These effects occurred at plasma tedizolid exposure levels (AUC) ≥3-fold greater than the expected human plasma exposure associated with the therapeutic dose.
14 CLINICAL STUDIES
14.1 Acute Bacterial Skin and Skin Structure Infections
A total of 1315 adults with acute bacterial skin and skin structure infections (ABSSSI) were randomized in two multicenter, multinational, double-blind, non-inferiority trials. Both trials compared SIVEXTRO 200 mg once daily for 6 days versus linezolid 600 mg every 12 hours for 10 days. In Trial 1, patients were treated with oral therapy, while in Trial 2, patients could receive oral therapy after a minimum of one day of intravenous therapy. Patients with cellulitis/erysipelas, major cutaneous abscess, or wound infection were enrolled in the trials. Patients with wound infections could have received aztreonam and/or metronidazole as adjunctive therapy for gram-negative bacterial coverage, if needed. The intent-to-treat (ITT) patient population included all randomized patients.
In Trial 1, 323 patients with ABSSSI were randomized to SIVEXTRO and 326 patients were randomized to linezolid. The majority (91%) of patients treated with SIVEXTRO in Trial 1 were less than 65 years old with a median age of 43 years (range: 18 to 86 years). Patients treated with SIVEXTRO were predominantly male (61%) and White (85%); 11% had BMI ≥35 kg/m2, 7% had diabetes mellitus, 36% were current or recent intravenous drug users, and 3% had moderate to severe renal impairment. The overall median surface area of infection was 190 cm2. The types of ABSSSI included were cellulitis/erysipelas (40%), wound infection (30%), and major cutaneous abscess (30%). In addition to local signs and symptoms of infection, patients were also required to have at least one regional or systemic sign of infection at baseline, defined as lymphadenopathy (87% of patients), temperature 38°C or higher (16% of patients), white blood cell count greater than 10,000 cells/mm3 or less than 4000 cells/mm3 (43%), or 10% or more band forms on white blood cell differential (4%).
The primary endpoint in Trial 1 was early clinical response defined as no increase from baseline lesion area at 48-72 hours after the first dose and oral temperature of ≤37.6°C, confirmed by a second temperature measurement within 24 hours in the ITT population.
In Trial 2, 332 patients with ABSSSI were randomized to SIVEXTRO and 334 patients were randomized to linezolid. The majority (87%) of patients treated with SIVEXTRO in Trial 2 were less than 65 years old with a median age of 46 years (range: 17 to 86 years). Patients treated with SIVEXTRO were predominantly male (68%) and White (86%); 16% had BMI ≥35 kg/m2, 10% had diabetes mellitus, 20% were current or recent intravenous drug users, and 5% had moderate to severe renal impairment. The overall median surface area of infection was 231 cm2. The types of ABSSSI included were cellulitis/erysipelas (50%), wound infection (30%), and major cutaneous abscess (20%). In addition to local signs and symptoms of infection, patients were also required to have at least one regional or systemic sign of infection at baseline, defined as lymphadenopathy (71% of patients), temperature 38°C or higher (31% of patients), white blood cell count greater than 10,000 cells/mm3 or less than 4000 cells/mm3 (53%), or 10% or more band forms on white blood cell differential (16%).
The primary endpoint in Trial 2 was early clinical response defined as at least a 20% decrease from baseline lesion area at 48-72 hours after the first dose in the ITT population (Table 7).
| SIVEXTRO (200 mg) |
Linezolid (1200 mg) |
Treatment Difference (2-sided 95% CI) |
|
|---|---|---|---|
| CI=confidence interval | |||
|
No increase in lesion surface area from baseline and oral temperature of ≤37.6°C, confirmed by a second temperature measurement within 24 hours at 48-72 hours |
|||
| Trial 1, N | 323 | 326 | |
| Responder, n (%) | 256 (79.3) | 258 (79.1) | 0.1 (-6.2, 6.3) |
| Trial 2, N | 332 | 334 | |
| Responder, n (%) | 286 (86.1) | 281 (84.1) | 2.0 (-3.5, 7.3) |
|
At least a 20% decrease from baseline in lesion area at 48-72 hours |
|||
| Trial 1, N | 323 | 326 | |
| Responder, n (%) | 252 (78.0) | 246 (75.5) | 2.6 (-4.0, 9.1) |
| Trial 2, N | 332 | 334 | |
| Responder, n (%) | 283 (85.2) | 276 (82.6) | 2.6 (-3.0, 8.2) |
An investigator assessment of clinical response was made at the post-therapy evaluation (PTE) (7 - 14 days after the end of therapy) in the ITT and CE (Clinically Evaluable) populations. Clinical success was defined as resolution or near resolution of most disease-specific signs and symptoms, absence or near resolution of systemic signs of infection if present at baseline (lymphadenopathy, fever, >10% immature neutrophils, abnormal WBC count), and no new signs, symptoms, or complications attributable to the ABSSSI requiring further treatment of the primary lesion (Table 8).
| SIVEXTRO (200 mg) n/N (%) |
Linezolid (1200 mg) n/N (%) |
Treatment Difference (2-sided 95% CI) |
|
|---|---|---|---|
| CI=confidence interval; ITT=intent-to-treat; CE=clinically evaluable | |||
| Trial 1 | |||
| ITT | 277/323 (85.8) | 279/326 (85.6) | 0.2 (-5.3, 5.6) |
| CE | 257/270 (95.2) | 260/273 (95.2) | -0.0 (-3.9, 3.7) |
| Trial 2 | |||
| ITT | 292/332 (88.0) | 293/334 (87.7) | 0.3 (-4.8, 5.3) |
| CE | 268/290 (92.4) | 269/280 (96.1) | -3.7 (-7.7, 0.2) |
Clinical success by baseline pathogens from the primary infection site or blood cultures for the microbiological intent-to-treat (MITT) patient population for two integrated Phase 3 ABSSSI studies are presented in Table 9 and Table 10.
| Pathogen | No increase in lesion surface area from baseline and oral temperature of ≤37.6°C |
At least a 20% decrease from baseline in lesion area |
||
|---|---|---|---|---|
| SIVEXTRO (200 mg) n/N (%) |
Linezolid (1200 mg) n/N (%) |
SIVEXTRO (200 mg) n/N (%) |
Linezolid (1200 mg) n/N (%) |
|
| Pooled analysis; n=number of patients in the specific category; N=Number of patients with the specific pathogen isolated from the ABSSSI | ||||
| Baseline bacteremia in the tedizolid arm with relevant pathogens included two subjects with MRSA, four subjects with MSSA, two subjects with S. pyogenes, and one subject with S. constellatus. All of these subjects were Responders at the 48-72 hour evaluation. At the Post-therapy Evaluation (PTE), 7 of 9 subjects were considered clinical successes. | ||||
| Staphylococcus aureus | 274/327 (83.8) | 276/339 (81.4) | 279/327 (85.3) | 273/339 (80.5) |
| Methicillin-resistant S. aureus | 111/140 (79.3) | 112/144 (77.8) | 114/140 (81.4) | 109/144 (75.7) |
| Methicillin-susceptible S. aureus | 163/187 (87.2) | 166/197 (84.3) | 165/187 (88.2) | 166/197 (84.3) |
| Streptococcus pyogenes | 27/33 (81.8) | 18/20 (90.0) | 25/33 (75.8) | 16/20 (80.0) |
| Streptococcus anginosus Group | 22/30 (73.3) | 26/28 (92.9) | 22/30 (73.3) | 25/28 (89.3) |
| Streptococcus agalactiae | 6/9 (66.7) | 8/10 (80.0) | 6/9 (66.7) | 7/10 (70.0) |
| Enterococcus faecalis | 7/10 (70.0) | 3/4 (75.0) | 6/10 (60.0) | 1/4 (24.0) |
| Pathogen | Clinical Response at PTE | |
|---|---|---|
| SIVEXTRO (200 mg) n/N (%) |
Linezolid (1200 mg) n/N (%) |
|
| Pooled analysis; n=number of patients in the specific category; N=Number of patients with the specific pathogen isolated from the ABSSSI | ||
| Baseline bacteremia in the tedizolid arm with relevant pathogens included two subjects with MRSA, four subjects with MSSA, two subjects with S. pyogenes, and one subject with S. constellatus. All of these subjects were Responders at the 48-72 hour evaluation. At the Post-therapy Evaluation (PTE) 7 of 9 subjects were considered clinical successes. | ||
| Staphylococcus aureus | 290/327 (88.7) | 300/339 (88.5) |
| Methicillin-resistant S. aureus | 118/140 (84.3) | 117/144 (81.3) |
| Methicillin-susceptible S. aureus | 172/187 (92.0) | 185/197 (93.9) |
| Streptococcus pyogenes | 30/33 (90.9) | 19/20 (95.0) |
| Streptococcus anginosus Group | 21/30 (70.0) | 25/28 (89.3) |
| Streptococcus agalactiae | 8/9 (88.9) | 8/10 (80.0) |
| Enterococcus faecalis | 7/10 (70.0) | 4/4 (100.0) |
15 REFERENCES
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard – 9th ed., CLSI document M7 A9. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard – 11th ed. CLSI document M2 A11 (ISBN 1-56238-781-2 [Print]; ISBN 1-56238-782-0 [Electronic]). Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087 USA, 2012.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing – 24th Informational Supplement. CLSI document M100 S24 (ISBN 1-56238-865-7 [Print]; ISBN 1-56238-866-5 [Electronic]). Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087 USA, 2014.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 Tablets
SIVEXTRO tablets are yellow film-coated oval tablets containing 200 mg of tedizolid phosphate; each tablet is debossed with "TZD" on one side and "200" on the other side.
They are supplied as follows:
HDPE bottles of 30 tablets with child-resistant closure (NDC 67919-041-01)
Unit dose blister packs of 6 tablets (NDC 67919-041-02)
16.2 For Injection
SIVEXTRO is supplied as a sterile, lyophilized powder for injection in single-use vials of 200 mg. Each 200 mg vial must be reconstituted with Sterile Water for Injection and subsequently diluted only with 0.9% Sodium Chloride Injection, USP.
They are supplied as follows:
Package of ten 200 mg single-dose vials (NDC 67919-040-01)
16.3 Storage and Handling
SIVEXTRO tablets and SIVEXTRO for injection should be stored at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Administration with Food
Patients should be informed that SIVEXTRO tablets may be taken with or without food and without any dietary restrictions [see Dosage and Administration (2.1) and Clinical Pharmacology (12.3) ].
Usage Safeguards
Patients should be advised that antibacterial drugs including SIVEXTRO should only be used to treat bacterial infections. SIVEXTRO does not treat viral infections (e.g., the common cold). When SIVEXTRO is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by SIVEXTRO or other antibacterial drugs in the future [see Indications and Usage (1.2) ].
Patients should be informed that if they miss a dose, they should take the dose as soon as possible anytime up to 8 hours prior to their next scheduled dose. If less than 8 hours remains before the next dose, then they should wait until their next scheduled dose. Patients should take the prescribed number of doses [see Dosage and Administration (2.1) ].
Keep SIVEXTRO and all medications out of reach of children.
Potentially Serious Adverse Reactions
Patients should be advised that diarrhea is a common problem caused by antibacterial drugs including SIVEXTRO and usually resolves when the drug is discontinued. Sometimes after starting treatment with antibiotics, patients can develop frequent watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic and may be a sign of a more serious intestinal infection [see Warnings and Precautions (5.2) and Adverse Reactions (6.1) ]. If this occurs, patients should contact their healthcare provider as soon as possible.
Distributed by:
Cubist Pharmaceuticals U.S.
Lexington, MA 02421 USA
Trademarks depicted herein are the property of their respective owners.
©2014 Cubist Pharmaceuticals
All rights reserved.
PRINCIPAL DISPLAY PANEL - 200 mg Tablet Blister Pack Carton
NDC 67919-041-02
6 tablets
Sivextro™
(tedizolid phosphate) tablets
200 mg per tablet
Rx only
CUBIST

PRINCIPAL DISPLAY PANEL - 200 mg Vial Carton
NDC 67919-040-01
10 single-use vials
Sivextro™
(tedizolid phosphate) for injection
200 mg per vial
For Intravenous Infusion
Rx only
Sterile
CUBIST

SIVEXTROtedizolid phosphate TABLET, COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SIVEXTROtedizolid phosphate POWDER, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||