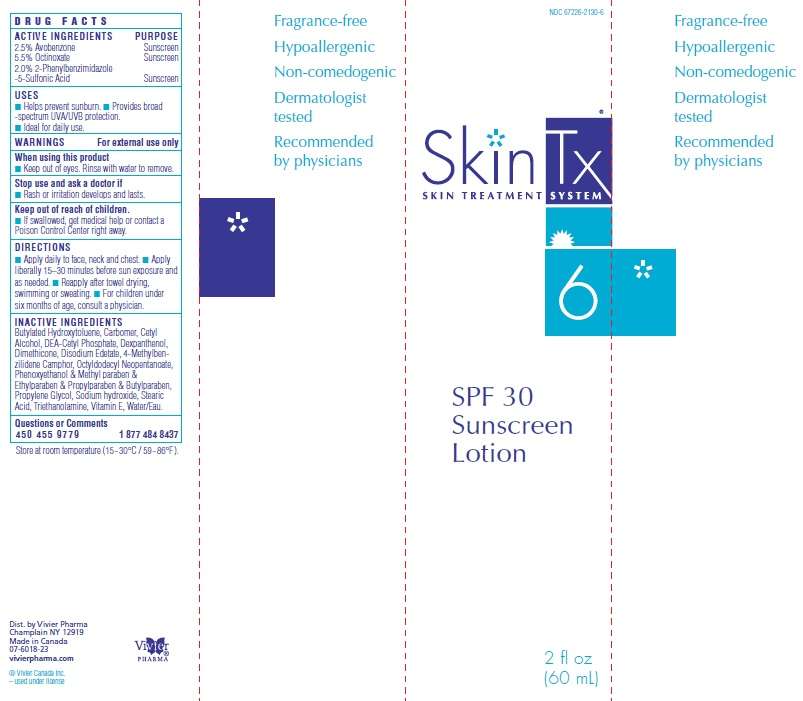
SkinTx Sunscreen
SkinTx SPF 30 Sunscreen Lotion
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- SkinTx Sunscreen Uses
- Warnings
- Directions
- Inactive Ingredients
- Questions or Comments
FULL PRESCRIBING INFORMATION
Active Ingredients
2.5% Avobenzone
5.5% Octinoxate
2.0% 2-Phenylbenzimidazole-5-Sulfonic Acid
Purpose
Sunscreen
SkinTx Sunscreen Uses
■ Helps prevent sunburn.
■ Provides broad -spectrum UVA/UVB protection.
■ Ideal for daily use.
Warnings
For external use only
When using this product
Keep out of eyes. Rinse with water to remove
Stop use and ask a doctor if
rash or irritation develops and lasts
Keep out of reach of children
■ If swallowed, get medical help or contact a Poison Control Center right away
Directions
■ Apply daily to face, neck and chest.
■ Apply liberally 15–30 minutes before sun exposure and as needed.
■ Reapply after towel drying, swimming or sweating.
■ For children under six months of age, consult a physician.
Inactive Ingredients
Butylated Hydroxytoluene, Carbomer, Cetyl Alcohol, DEA-Cetyl Phosphate, Dexpanthenol, Dimethicone, Disodium Edetate, 4-Methylbenzilidene Camphor, Octyldodecyl Neopentanoate, Phenoxyethanol and Methyl paraben and Ethylparaben and Propylparaben and Butylparaben, Propylene Glycol, Sodium hydroxide, Stearic Acid, Triethanolamine, Vitamin E, Water/Eau.
Questions or Comments
450 455 9779 1 877 484 8437
Store at room temperature (15-30°C / 59-86°F).
Fragrance-free
Hypoallergenic
Non-comedogenic
Dermatologist tested
Recommended by physicians
NDC 67226-2130-6
SkinTx
Skin Treatment System 6
SPF 30
Sunscreen
Lotion
2 fl oz
(60 mL)
Dist. by Vivier Pharma
Champlain NY 12919
Made in Canada
07-6018-23
vivierpharma.com
® Vivier Canada Inc.
– used under license

SkinTx SunscreenAVOBENZONE, OCTINOXATE, ENSULIZOLE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||