Sodium Chloride Hypertonicity
Sodium Chloride Hypertonicity Ophthalmic Solution Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients
- Purpose
- Sodium Chloride Hypertonicity Uses
- Warnings
- Directions
- Sodium Chloride Hypertonicity Other information
- Inactive ingredient
- Questions?
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredients
Sodium chloride 5%
Purpose
Hypertonicity agent
Sodium Chloride Hypertonicity Uses
temporary relieve of corneal edema
Warnings
Do not use
- except under the advice and supervision of a doctor
- if solution changes color or becomes cloudy
When using this product
- it may cause temporary burning and irritation
- do not touch tip of container to any surface to avoid contamination
- replace cap after use
Stop use and ask a doctor if
- condition worsens or persists for more than 72 hours
- you experience eye pain, changes in vision, continued redness or irritation of the eye
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
-
Sodium Chloride Hypertonicity Other information
- store upright at 15° - 30°C (59° - 86°F)
- keep tightly closed
Inactive ingredient
boric acid, hypromellose, propylene glycol, purified water, sodium borate. Sodium hydroxide ad/or hydrochloric acid may be added to adjust pH. PRESERVATIVE ADDED: methylparaben 0.023%, propylparaben 0.01%
Questions?
Serious side effects associated with use of this product may be reported to 1-800-323-0000
Package/Label Principal Display Panel
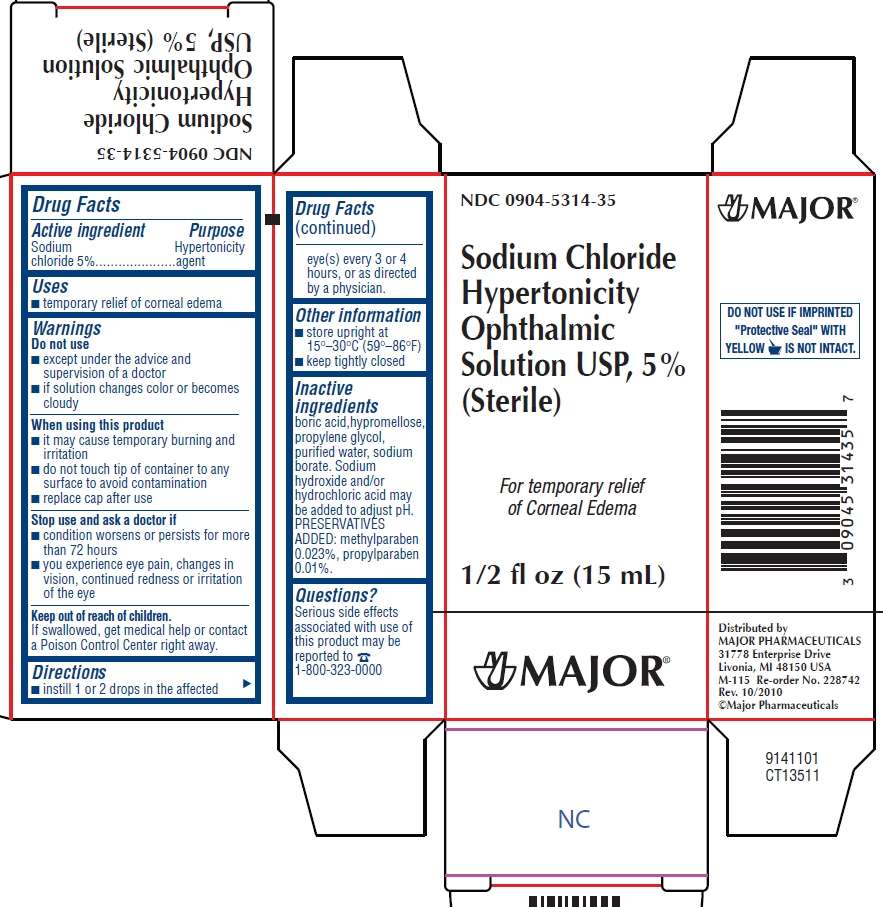
NDC 0904-5314-35
Sodium Chloride Hypertonicity Ophthalmic Solution USP, 5%
(Sterile)
For temporary relief of Corneal Edema
1/2 fl oz (15mL)
MAJOR®
Sodium Chloride Hypertonicitysodium chloride SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||