Sodium Chloride
Sodium Chloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- HEALTH CARE PROVIDER LETTER
- SODIUM CHLORIDE DESCRIPTION
- PHARMACOLOGY
- INDICATIONS
- SODIUM CHLORIDE CONTRAINDICATIONS
- PRECAUTIONS
- USE IN PREGNANCY
- USE IN LACTATION
- PAEDIATRIC USE
- USE IN ELDERLY
- INTERACTIONS
- SODIUM CHLORIDE ADVERSE REACTIONS
- SODIUM CHLORIDE DOSAGE AND ADMINISTRATION
- OVERDOSAGE
- TREATMENT
- PRESENTATION AND STORAGE CONDITIONS
FULL PRESCRIBING INFORMATION
HEALTH CARE PROVIDER LETTER
| 0.9% SODIUM CHLORIDE INJECTION SOLUTION AVAILABILITY |
Subject: Importation of European Drug Product
March 3, 2014
Dear Healthcare Professional,
Due to the current critical shortage of 0.9% Sodium Chloride Injection in the U.S. market, Fresenius Kabi USA, LLC (Fresenius Kabi USA) is coordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of the drug. Fresenius Kabi USA has initiated temporary importation of a European Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion into the U.S. market. The European product contains the same active ingredient in the same concentration as the 0.9% Sodium Chloride Injection products approved in the United States. The Sodium Chloride 0.9% Freeflex Injection Solution product is manufactured near Halden, Norway, at Fresenius Kabi Norge AS, an FDA inspected facility. At its most recent FDA inspection, this facility was found to be in compliance with current good manufacturing practices.
At this time, FDA is not objecting to the importation and distribution of Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion, by Fresenius Kabi USA, to address the critical shortage of 0.9% Sodium Chloride Injection. Importation or distribution of Fresenius Kabi’s Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion by any entity other than Fresenius Kabi USA, is not within the scope of this decision and may be subject to enforcement action by the FDA. FDA has not approved Fresenius Kabi’s Sodium Chloride 0.9% Freeflex Injection Solution products in the United States.
Effective immediately, and during this temporary period, Fresenius Kabi USA will offer the following presentations of Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion:
|
Fresenius Kabi’s Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion |
||
|
Product Name |
Volume |
Ingredients |
|
Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion |
50 mL |
Each 50 mL contains: Sodium Chloride 450 mg, Water for Injections to 50 mL
Total Electrolytes per 50 mL approx: Sodium 7.7 mmol*, Chloride 7.7 mmol* |
|
Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion |
100 mL |
Each 100 mL contains: Sodium Chloride 900 mg, Water for Injections to 100 mL
Total Electrolytes per 100 mL approx: Sodium 15.4 mmol*, Chloride 15.4 mmol* |
|
Sodium Chloride 0.9% w/v for Intravenous Infusion |
500 mL |
Each 500 mL contains: Sodium Chloride 4.5 g, Water for Injections to 500 mL
Total Electrolytes per 500 mL approx: Sodium 77 mmol*, Chloride 77 mmol* |
|
Sodium Chloride 0.9% w/v for Intravenous Infusion |
1000 mL |
Each 1000 mL contains: Sodium Chloride 9 g, Water for Injections to 1000 mL
Total Electrolytes per 1000 mL approx: Sodium 154 mmol*, Chloride 154 mmol* |
* For monovalent ions, such as sodium and chloride, the numeric value of the millimole and milliequivalent are identical.
Fresenius Kabi’s Sodium Chloride 0.9% Injection Solution for Intravenous Infusion is packaged in free flex ®, a flexible bag with self-sealing ports and made of multilayer Polyolefin film that is PVC-free, plasticizer-free, latex-free and non-DEHP.

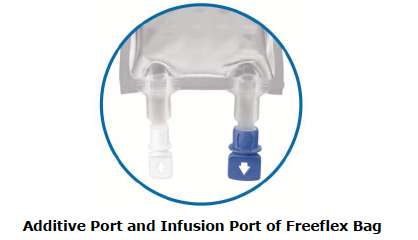
It is important to note that there are differences in the formatting and content of the labeling between the U.S. marketed 0.9% Sodium Chloride solutions, and Fresenius Kabi’s Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion. Refer to comparison table attached.
|
Differences Between U.S. Prescribing Information and Fresenius Kabi’s Prescribing Information |
||
|
Property |
0.9% Sodium Chloride U.S. Products |
Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion |
|
Active Ingredients |
Sodium 154 mEq/L* |
Sodium 154 mmol/L* |
|
Chloride 154 mEq/L* |
Chloride 154 mmol/L* |
|
|
Osmolarity 308 mOsmol/L (calc.) |
Osmolality 308 mOsmol/kg water** (calc.) |
|
|
Indications for Use |
See manufacturer’s package insert |
Normal saline can be used as the vehicle for many parenteral drugs and as a sterile irrigation medium. |
|
Contraindications |
See manufacturer’s package insert |
Sodium Chloride 0.9% is contraindicated in patients with congestive heart failure, severe renal impairment, conditions of sodium retention, edema, liver cirrhosis and irrigation during electrosurgical procedures. |
|
Warnings |
Caution must be exercised in the administration of parenteral fluids, especially those containing sodium ions to patients receiving corticosteroids or corticotropin. |
Refer to Fresenius Kabi’s package insert or visit www.fresenius-kabi.us |
|
Adverse Reactions |
See manufacturer’s package insert |
Excessive amounts of sodium chloride may cause hypernatremia, hypokalemia and acidosis. Proper use of normal saline as a vehicle for parenteral drugs or as an electrolyte replacement therapy is unlikely to result in adverse effects.
Hypernatremia rarely occurs with therapeutic doses of sodium chloride, but may occur in excessive administration. A serious complication of this is dehydration of the brain causing somnolence and confusion, which may progress to convulsions, coma and ultimately respiratory failure and death. Pulmonary embolism or pneumonia may also result. Other symptoms include thirst, reduced salivation and lacrimation, fever, tachycardia, hypertension, headache, dizziness, restlessness, weakness and irritability.
Displaced catheters or drainage tubes can lead to irrigation or infiltration of unintended structures or cavities. Excessive volume or pressure during irrigation of closed cavities may result in distension or disruption of tissues. Inadvertent contamination from careless technique may transmit infection. Adverse effects resulting from irrigation of body cavities, tissues or indwelling catheters and tubes are usually avoidable when appropriate procedures are followed. |
|
Drug Interactions |
See manufacturer’s package insert |
Co-medication of drugs inducing sodium retention may exacerbate any systemic effects. |
|
Overdose and Treatment |
See manufacturer’s package insert |
Overdose Infusion of excess intravenous fluid may cause hypervolemia and electrolyte imbalances. Excess sodium chloride in the body produces general gastrointestinal effects of nausea, vomiting, diarrhea and cramps. Salivation and lacrimation are reduced, while thirst and sweating are increased. Hypotension, tachycardia, renal failure, peripheral and pulmonary edema and respiratory arrest may occur. CNS symptoms include headache, dizziness, restlessness, irritability, weakness, muscular twitching and rigidity, convulsions, coma and death. If any adverse effects are observed during administration, discontinue infusion, evaluate the patient and institute appropriate supportive treatment.
Treatment Normal plasma sodium concentrations should be carefully restored at a rate not greater than 10-15 mmol/day using I.V. hypotonic saline. Dialysis may be necessary if there is significant renal impairment, the patient is moribund or plasma sodium levels are greater than 200 mmol/L. Convulsions may require diazepam or other appropriate treatment. |
|
Container Type |
See manufacturer’s package insert |
Packaged in free flex ® bag. Made of multilayer polyolefin film. Non-DEHP, non-PVC, and latex-free. |
|
Barcode |
Readable U.S. barcodes |
Any barcodes on Fresenius Kabi’s Sodium Chloride 0.9% free flex ® solution will not be appropriately recognized by scanning systems used in the United States and should not be used. Institutions should manually input the product into their systems and confirm that barcode systems do not provide incorrect information when the product is scanned. |
* For monovalent ions, such as sodium and chloride, the numeric value of the millimole and milliequivalent are identical
** 1 kilogram of water is equal to 1 liter of water
|
Refer to the package insert for Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion for full prescribing information |
This communication and product information is available on the Fresenius Kabi USA web site www.fresenius-kabi.us as well as on the FDA Drug Shortage web site. http://www.fda.gov/Drugs/DrugSafety/DrugShortages/default.htm .
REPORTING ADVERSE EVENTS:
To report adverse events or quality problems experienced with the use of this product, call Fresenius Kabi USA Vigilance and Medical Affairs at 1-800-551-7176, Monday - Friday, between the hours of 8 a.m. and 5 p.m. (CST), or e-mail appmedicalinfo@APPpharma.com.
Fresenius Kabi USA CONTACT NUMBERS: Please use the following contact numbers as appropriate:
|
Reason To Call |
Department |
Number |
|
ADE Reporting/Clinical/Technical Info. |
Vigilance and Medical Affairs Dept. |
1-800-551-7176 |
|
Product Availability & Ordering |
Customer Service Department |
1-888-386-1300 |
Adverse events may also be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Online : www.fda.gov/medwatch/report.htm
- Regular Mail : use postage-paid FDA form 3500 available at: www.fda.gov/MedWatch/getforms.htm . Mail to: MedWatch 5600 Fishers Lane, Rockville, MD 20852-9787
- Fax : 1-800-FDA-0178
Sincerely,
Melanie Power-Burns
Senior Director, U.S. Quality & Compliance
Comparison Table of U.S. 0.9% Sodium Chloride Injection to Fresenius Kabi’s Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion
|
Fresenius Kabi |
Hospira |
Hospira |
Hospira |
Baxter |
Baxter |
Baxter |
|||
|
|
|
|
|
|
|
Product Image Not Available |
|
||
|
Product |
Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion |
0.9% Sodium Chloride Injection, USP |
0.9% Sodium Chloride Injection, USP |
0.9% Sodium Chloride Injection, USP |
0.9% Sodium Chloride Injection, USP |
0.9% Sodium Chloride Injection, USP |
0.9% Sodium Chloride Injection, USP |
||
|
NDC # |
63323-623-50 |
0409-7984-06 |
0409-7984-13 |
0409-7984-36 |
0338-0049-11 |
0338-0049-31 |
0338-0049-41 |
||
|
Volume |
50 mL |
50 mL |
50 mL |
50 mL |
50 mL |
50 mL |
50 mL |
||
|
Container Type |
free flex ® |
VisIV™ Flexible plastic container |
Flexible plastic container |
Flexible plastic container |
VIAFLEX plastic container |
VIAFLEX plastic container |
VIAFLEX plastic container |
||
|
Container Description |
Made of multilayer polyolefin film. Non-DEHP, non-PVC, and latex free. |
Fabricated from a clear mutilayer polyolefin plastic film. PVC, DEHP, and latex free. |
PVC container. Contains DEHP, latex free. |
PVC container. Contains DEHP, latex free. |
PVC container. Contains DEHP, non-latex. |
PVC container. Contains DEHP, non-latex. |
PVC container. Contains DEHP, non-latex. |
||
|
Preservative Free |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
||
|
Case Size |
60 |
60 |
48 |
80 (Quad Pack) |
96 (Quad Pack) |
96 (Multi Pack) |
96 |
||
|
Fresenius Kabi |
Hospira |
Hospira |
Hospira |
Baxter |
Baxter |
Baxter |
|||
|
|
|
|
|
|
|
|
|
||
|
Product |
Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion |
0.9% Sodium Chloride Injection, USP |
0.9% Sodium Chloride Injection, USP |
0.9% Sodium Chloride Injection, USP |
0.9% Sodium Chloride Injection, USP |
0.9% Sodium Chloride Injection, USP |
0.9% Sodium Chloride Injection, USP |
||
|
NDC # |
63323-623-00 |
0409-7984-11 |
0409-7984-23 |
0409-7984-37 |
0338-0049-18 |
0338-0049-38 |
0338-0049-48 |
||
|
Volume |
100 mL |
100 mL |
100 mL |
100 mL |
100 mL |
100 mL |
100 mL |
||
|
Container Type |
free flex ® |
VisIV™ Flexible plastic container |
Flexible plastic container |
Flexible plastic container |
VIAFLEX plastic container |
VIAFLEX plastic container |
VIAFLEX plastic container |
||
|
Preservative Free |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
||
|
Container Description |
Made of multilayer polyolefin film. Non-DEHP, non-PVC, and latex free. |
Fabricated from a clear mutilayer polyolefin plastic film. PVC, DEHP, and latex free. |
PVC container. Contains DEHP, latex free. |
PVC container. Contains DEHP, latex free. |
PVC container. Contains DEHP, non-latex. |
PVC container. Contains DEHP, non-latex. |
PVC container. Contains DEHP, non-latex. |
||
|
Case Size |
50 |
60 |
48 |
80 (Quad Pack) |
96 (Quad Pack) |
96 (Multi Pack) |
96 |
||
|
Fresenius Kabi |
Hospira |
Hospira |
Baxter |
|
|
|
|
|
|
|
|
Product |
Sodium Chloride 0.9% w/v for Intravenous Infusion |
0.9% Sodium Chloride Injection, USP |
0.9% Sodium Chloride Injection, USP |
0.9% Sodium Chloride Injection, USP |
|
NDC # |
63323-623-59 |
0409-7983-03 |
0409-7983-55 |
0338-0049-03 |
|
Volume |
500 mL |
500 mL |
500 mL |
500 mL |
|
Container Type |
free flex ® |
Flexible plastic container |
Flexible plastic container |
VIAFLEX plastic container |
|
Preservative Free |
Yes |
Yes |
Yes |
Yes |
|
Container Description |
Made of multilayer polyolefin film. Non-DEHP, non-PVC, and latex free. |
PVC container. Contains DEHP, latex free. |
PVC container. Contains DEHP, latex free. |
PVC container. Contains DEHP, non-latex. |
|
Case Size |
20 |
24 |
18 |
24 |
|
Fresenius Kabi |
Hospira |
Baxter |
|
|
|
|
|
|
|
Product |
Sodium Chloride 0.9% w/v for Intravenous Infusion |
0.9% Sodium Chloride Injection, USP |
0.9% Sodium Chloride Injection, USP |
|
NDC # |
63323-623-10 |
0409-7983-09 |
0338-0049-04 |
|
Volume |
1000 mL |
1000 mL |
1000 mL |
|
Container Type |
free flex ® |
Flexible plastic container |
VIAFLEX plastic container |
|
Preservative Free |
Yes |
Yes |
Yes |
|
Container Description |
Made of multilayer polyolefin film. Non-DEHP, non-PVC, and latex free. |
Fabricated from a clear mutilayer polyolefin plastic film. PVC, DEHP, and latex free |
PVC container. Contains DEHP, non-latex. |
|
Case Size |
10 |
12 |
14 |

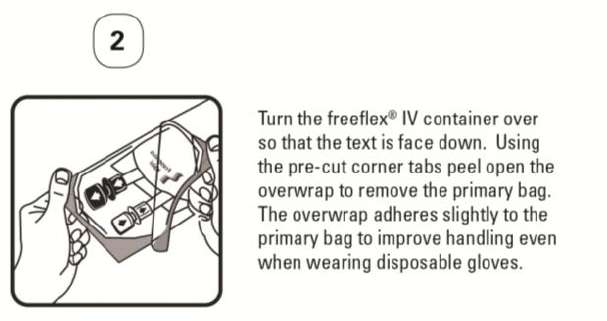
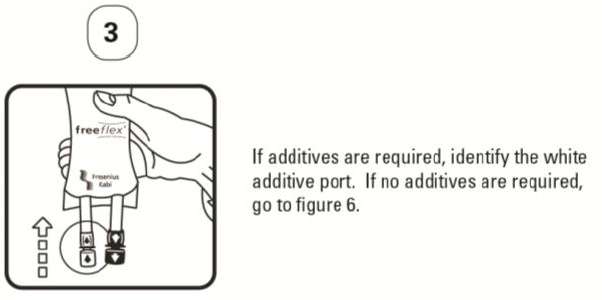
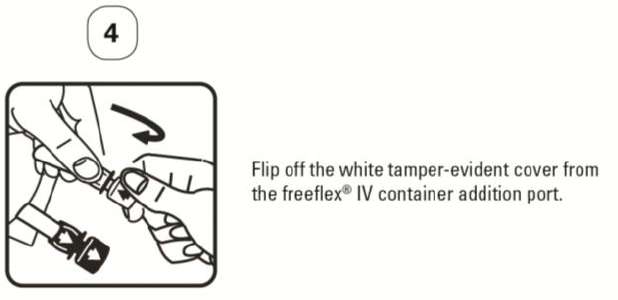
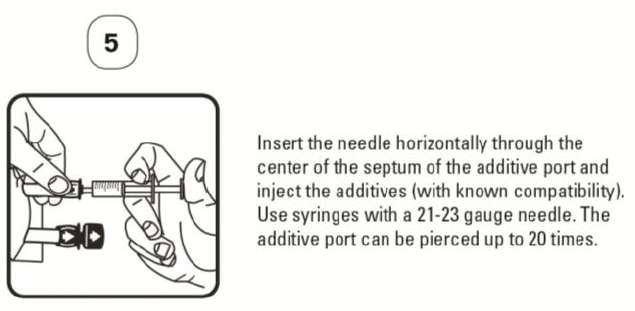
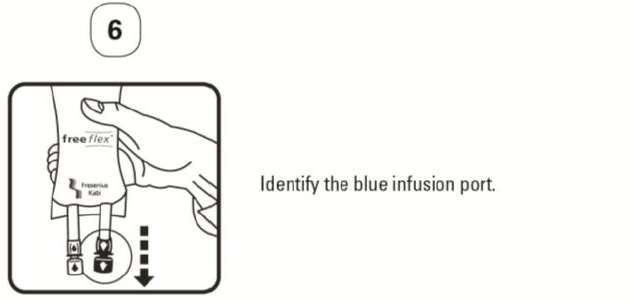
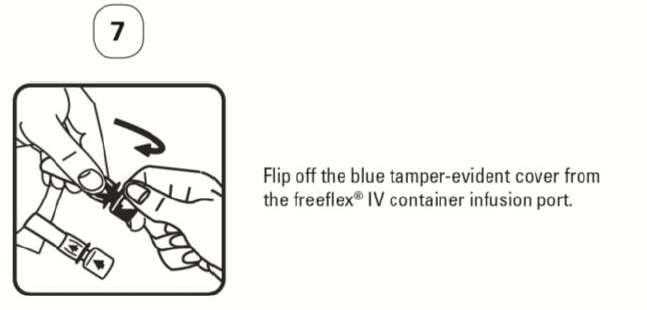
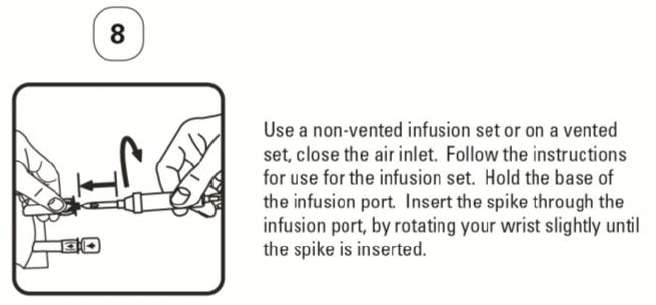

PATIENT LEAFLET: INFORMATION FOR THE USER
SODIUM CHLORIDE DESCRIPTION
Sterile isotonic solution of sodium chloride 9g/L in Water for Injections, containing no preservatives (normal saline).
PHARMACOLOGY
Sodium Chloride Injection 0.9% provides a source of sodium ions (154 mmol/L), chloride ions (154 mmol/L) and water.
INDICATIONS
Normal saline can be used as the vehicle for many parenteral drugs and as an electrolyte replenisher for maintenance or replacement of deficits of extracellular fluid.
It can also be used as a sterile irrigation medium.
SODIUM CHLORIDE CONTRAINDICATIONS
Sodium Chloride 0.9% is contraindicated in patients with congestive heart failure, severe renal impairment, conditions of sodium retention, oedema, liver cirrhosis and irrigation during electrosurgical procedures.
PRECAUTIONS
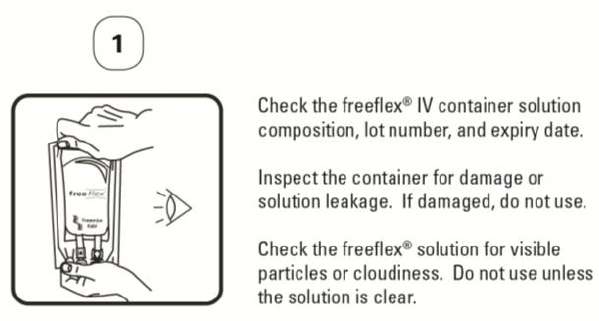
Do not use unless the solution is clear. The entire contents of the bag should be used promptly.
When used as a vehicle for intravenous drug delivery, the product information document of such drugs should be checked prior to use to ensure compatibility with the sodium chloride solution. Reconstitution instructions should be read carefully.
Excessive administration of sodium chloride causes hypernatraemia, resulting in dehydration of internal organs, hypokalaemia and acidosis. Monitoring of fluid, electrolyte and acid/base balance may be necessary. Congestive heart failure and pulmonary oedema may be precipitated, particularly in patients with cardiovascular disease or those receiving corticosteroids, corticotrophin or other drugs that may give rise to sodium retention. Sodium chloride should be administered with care to patients with congestive heart failure, hypertension, peripheral or pulmonary oedema, hypoproteinaemia, impaired renal function, urinary tract obstruction, pre-eclampsia and very young or elderly patients. Intravenous infusion during or immediately after surgery may result in sodium retention.
Given that there is a possibility of systemic absorption of irrigation solutions, the same precautions apply.
USE IN PREGNANCY
Safety in pregnancy has not been established. Use is recommended only when clearly indicated.
USE IN LACTATION
Safety in lactation has not yet been established. Use of this product while breastfeeding is recommended only when potential benefits outweigh potential risks to the newborn infant.
PAEDIATRIC USE
In paediatric use, the dose should be calculated for each patient based on clinical condition, including body weight and laboratory data.
USE IN ELDERLY
For use in elderly, the dose should be based on individual patient assessment, including weight, fluid and electrolyte status and renal and cardiac function.
INTERACTIONS
Additives may be incompatible with sodium chloride.
Co-medication of drugs inducing sodium retention may exacerbate any systemic effects.
SODIUM CHLORIDE ADVERSE REACTIONS
Excessive amounts of sodium chloride may cause hypernatraemia, hypokalaemia and acidosis. Proper use of normal saline as a vehicle for parenteral drugs or as an electrolyte replacement therapy is unlikely to result in adverse effects.
Hypernatraemia rarely occurs with therapeutic doses of sodium chloride, but may occur in excessive administration. A serious complication of this is dehydration of the brain causing somnolence and confusion, which may progress to convulsions, coma and ultimately respiratory failure and death. Pulmonary embolism or pneumonia may also result. Other symptoms include thirst, reduced salivation and lacrimation, fever, tachycardia, hypertension, headache, dizziness, restlessness, weakness and irritability.
Infusion of excess sodium chloride 0.9% solution may cause fluid overload or electrolyte imbalance. Intravenous administration of solutions may cause local reactions including pain, vein irritation and thrombophlebitis. Extravasation of solution may cause tissue injury.
If any adverse effects are observed during administration, discontinue infusion, evaluate the patient and institute appropriate supportive treatment.
Displaced catheters or drainage tubes can lead to irrigation or infiltration of unintended structures or cavities. Excessive volume or pressure during irrigation of closed cavities may result in distension or disruption of tissues. Inadvertent contamination from careless technique may transmit infection. Adverse effects resulting from irrigation of body cavities, tissues or indwelling catheters and tubes are usually avoidable when appropriate procedures are followed.
SODIUM CHLORIDE DOSAGE AND ADMINISTRATION
The dosage of sodium chloride as a vehicle for parenteral drugs and as an electrolyte replenisher must be calculated after consideration of clinical and laboratory data.
For use in one patient, on one occasion only. It does not contain antimicrobials. Any unused portion should be discarded. Care should be taken with intravenous technique to avoid injection site reactions and infections.
OVERDOSAGE
Infusion of excess intravenous fluid may cause hypervolaemia and electrolyte imbalances. Excess sodium chloride in the body produces general gastrointestinal effects of nausea, vomiting, diarrhoea and cramps. Salivation and lacrimation are reduced, while thirst and sweating are increased. Hypotension, tachycardia, renal failure, peripheral and pulmonary oedema and respiratory arrest may occur. CNS symptoms include headache, dizziness, restlessness, irritability, weakness, muscular twitching and rigidity, convulsions, coma and death. If any adverse effects are observed during administration, discontinue infusion, evaluate the patient and institute appropriate supportive treatment.
TREATMENT
Normal plasma sodium concentrations should be carefully restored at a rate not greater than 10-15 mmol/day using I.V. hypotonic saline. Dialysis may be necessary if there is significant renal impairment, the patient is moribund or plasma sodium levels are greater than 200 mmol/L. Convulsions may require diazepam or other appropriate treatment.
PRESENTATION AND STORAGE CONDITIONS
Freeflex bags - Store below 25°C.
50mL AUST R 144596
100mL AUST R 144609
250mL AUST R 144632
500mL AUST R 29745
1000mL AUST R 47400
Poison schedule of the medicine
Australia: Nil
New Zealand: General Sales Medicine
Date of Approval: 9th May 2005
Date of Most Recent Amendment : 16 July 2010
Name and address of the sponsor Fresenius Kabi Australia Pty Limited
964 Pacific Highway
Pymble NSW 2073 Australia
Tel: (02) 9391 5555
Fresenius Kabi New Zealand Limited
60 Pavilion Drive
Airport Oaks, Auckland 2022
New Zealand
Freecall: 0800 144 892
PACKAGE LABEL - PRINCIPAL DISPLAY - Sodium Chloride - 50 mL Bag
50 mL Code FAH3038
Sodium Chloride 0.9% Injection Solution
for Intravenous Infusion

PACKAGE LABEL - PRINCIPAL DISPLAY - Sodium Chloride - 100 mL Bag
100 mL Code FAH3015
Sodium Chloride 0.9% Injection Solution
for Intravenous Infusion

PACKAGE LABEL - PRINCIPAL DISPLAY - Sodium Chloride - 500 mL Bag
500 mL Code K690521
Sodium Chloride 0.9% w/v
for Intravenous Infusion

PACKAGE LABEL - PRINCIPAL DISPLAY - Sodium Chloride - 1000 mL Bag
1000 mL FAH1324
Sodium Chloride 0.9% w/v
for Intravenous Infusion

Sodium ChlorideSODIUM CHLORIDE INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||