Sodium Chloride
Preservative-Free 0.9% Sodium Chloride Injection, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- SODIUM CHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- SODIUM CHLORIDE INDICATIONS AND USAGE
- PRECAUTIONS
- SODIUM CHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- RL-0000
FULL PRESCRIBING INFORMATION
Rx only
SODIUM CHLORIDE DESCRIPTION
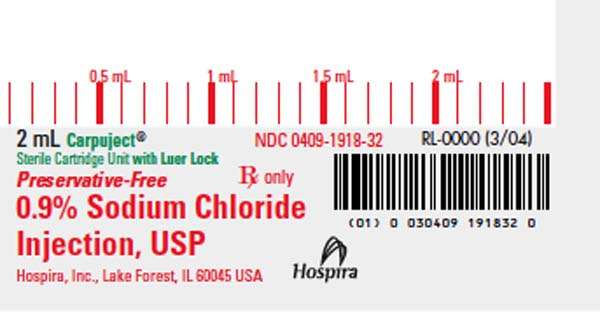
0.9% Sodium Chloride Injection, USP in Carpuject ™ sterile cartridge unit (2 mL, 3 mL, and 5 mL fill), is a sterile aqueous isotonic solution of sodium chloride in Water for Injection. The total osmolar concentration of the solution is 0.31 mOsm/mL. Each mL contains 9 mg of sodium chloride.
CLINICAL PHARMACOLOGY
0.9% Sodium Chloride Injection, USP is a sterile aqueous vehicle having approximately the same osmotic pressure and composition as extracellular fluids. It is nonirritating to tissues.
SODIUM CHLORIDE INDICATIONS AND USAGE
For use as a sterile isotonic vehicle for diluting or dissolving compatible parenteral medications.
PRECAUTIONS
If compatibility is in doubt, consult the appropriate specialized literature before mixing with any drug product. Do not administer if a precipitate appears, if solution is cloudy or hazy, or if any other visible change occurs upon mixing.
For single use only. Discard unused solution.
SODIUM CHLORIDE DOSAGE AND ADMINISTRATION
Use in accord with any warnings or precautions appropriate to the medication being administered.
HOW SUPPLIED
|
NDC No. |
Container |
Fill |
Quantity |
|
0409-1918-32 |
Carpuject™ with Luer Lock |
2 mL fill in 2 mL cartridge |
bins of 50 |
|
0409-1918-33 |
Carpuject™ with Luer Lock |
3 mL fill in 5 mL cartridge |
bins of 25 |
|
0409-1918-35 |
Carpuject™ with Luer Lock |
5 mL fill in 5 mL cartridge |
bins of 25 |
Do not use if solution is discolored or contains a precipitate.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature].
Do not freeze.
Revised: November, 2009
Printed in USA
EN-2305
Hospira, Inc., Lake Forest, IL 60045 USA
RL-0000
Sodium ChlorideSODIUM CHLORIDE INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||