Softlips Tint
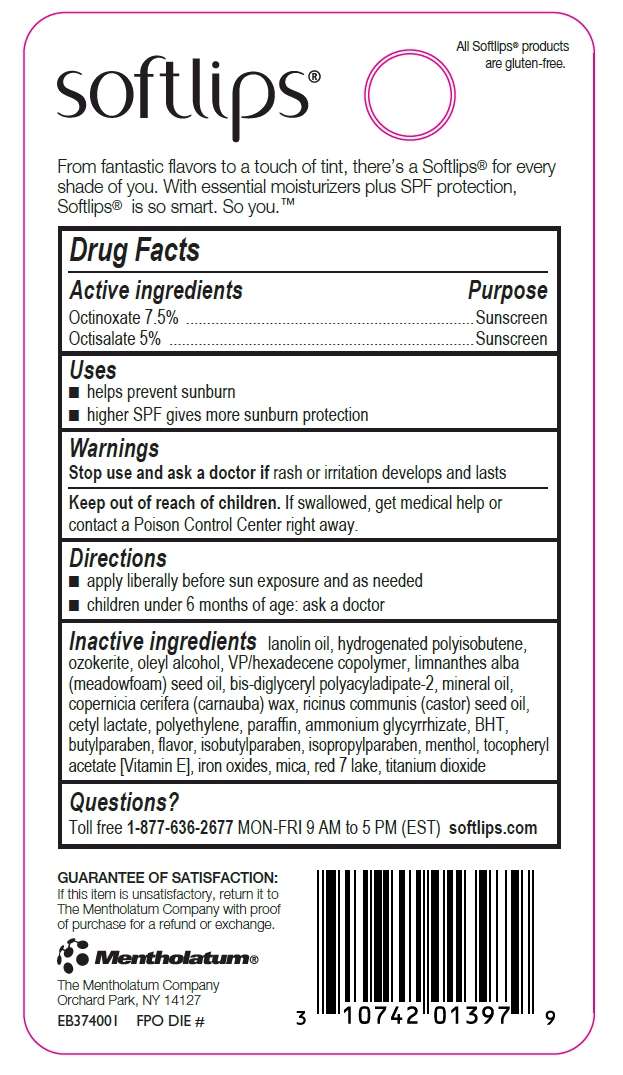
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients
- Purpose
- Softlips Tint Uses
- Warnings
- Directions
- Inactive ingredients
- Questions?
- Package/Label Principal Display Panel
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredients
Octinoxate 7.5%
Octisalate 5%
Purpose
Octinoxate - Sunscreen
Octisalate - Sunscreen
Softlips Tint Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
Warnings
Stop use and ask a doctor if
rash or irritation develops and lasts
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally before sun exposure and as needed
- children under 6 months of age: ask a doctor
Inactive ingredients
lanolin oil, hydrogenated polyisobutene, ozokerite, oleyl alcohol, VP/hexadecene copolymer, limnanthes alba (meadowfoam) seed oil, bis-diglyceryl polyacyladipate-2, mineral oil, copernica cerifera (carnauba) wax, ricinus communis (castor) seed oil, cetyl lactate, polyethylene, paraffin, ammonium glycyrrhizate, BHT, butylparaben, flavor, isobutylparaben, isopropylparaben, menthol, tocopheryl acetate, iron oxides, mica, red 7 lake, titanium dioxide
Questions?
Toll free 1-877-636-2677 MON - FRI 9 AM - 5 PM (EST) softlips.com
Package/Label Principal Display Panel

Softlips Rose Tint
SPF 15 tinted lip conditioner sunscreen
Package/Label Principal Display Panel
The Mentholatum Company
Orchard Park, NY 14127
Softlips TintOctinoxate, Octisalate LIPSTICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||