Softsoap Instant Hand Sanitizer with moisturizers
Softsoap Instant Hand Gel Sanitizer with moisturizers (800ml)
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
- Softsoap Instant Hand Sanitizer with moisturizers Other information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 800 mL Bottle Carton
FULL PRESCRIBING INFORMATION
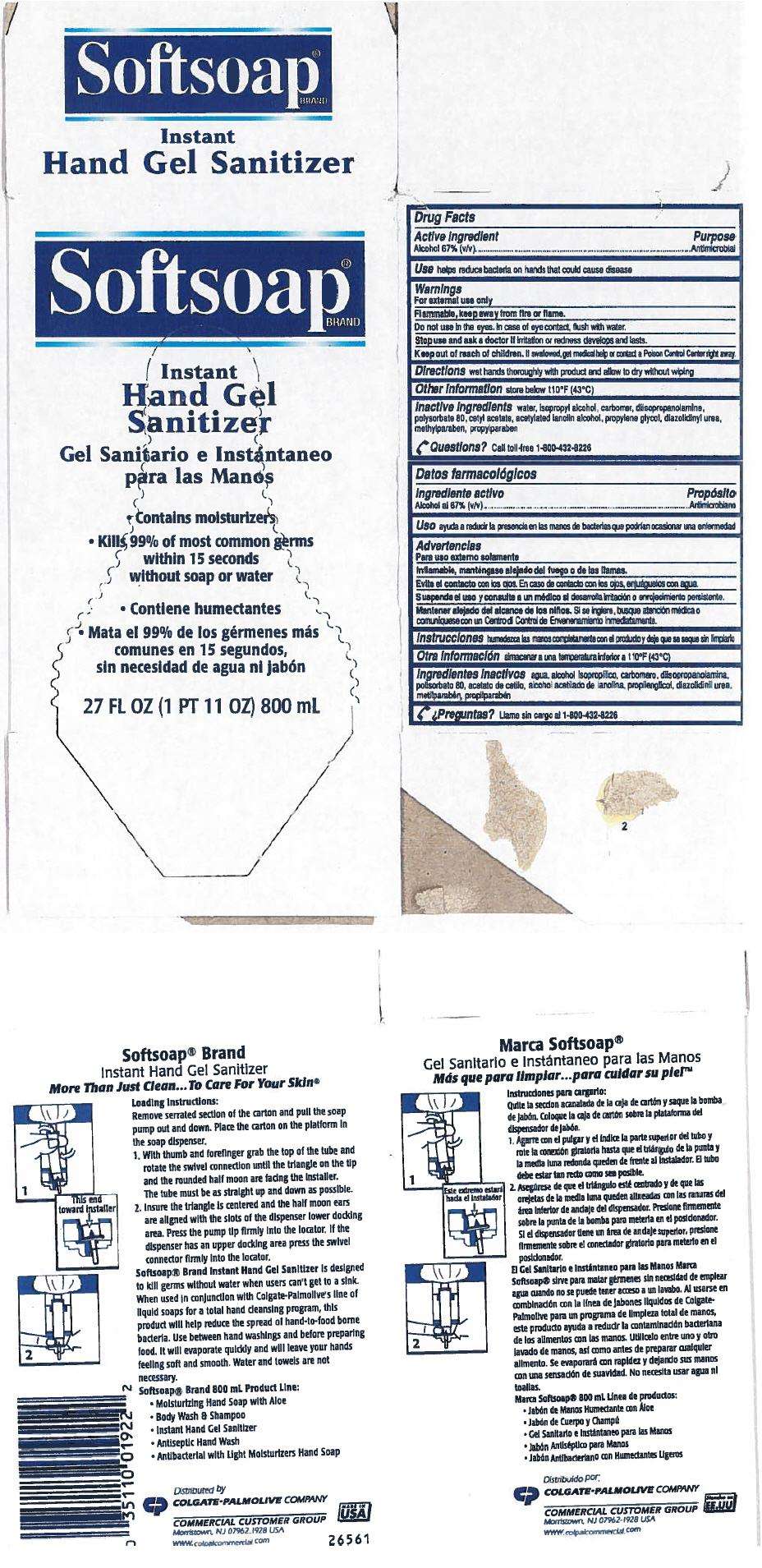
Drug Facts
Active Ingredient
Alcohol 67% (v/v)
Purpose
Antimicrobial
Use
helps reduce bacteria on hands that could cause disease
Warnings
For external use only
Flammable, keep away from fire or flame.
Do not use in the eyes. In case of eye contact, flush with water.
Stop use and ask a doctor if irritation or redness develops and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
wet hands thoroughly with product and allow to dry without wiping
Softsoap Instant Hand Sanitizer with moisturizers Other information
store below 110°F (43°C)
Inactive ingredients
water, isopropyl alcohol, carbomer, diisopropanolamine, polysorbate 80, cetyl acetate, acetylated lanolin alcohol, propylene glycol, diazolidinyl urea, methylparaben, propylparaben
Questions?
Call toll-free 1-800-432-8226
Distributed by
COLGATE-PALMOLIVE
COMPANY
COMMERCIAL CUSTOMER GROUP
Morristown, NJ 07962-1928 USA
PRINCIPAL DISPLAY PANEL - 800 mL Bottle Carton
Softsoap®
BRAND
Instant
Hand Gel
Sanitizer
- Contains moisturizers
- Kills 99% of most common germs
within 15 seconds
without soap or water
27 FL OZ (1 PT 11 OZ) 800 mL
Softsoap Instant Hand Sanitizer with moisturizersAlcohol GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||