Solu-Medrol
FULL PRESCRIBING INFORMATION: CONTENTS*
- SOLU-MEDROL DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- SOLU-MEDROL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- SOLU-MEDROL ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
SOLU-MEDROL DESCRIPTION
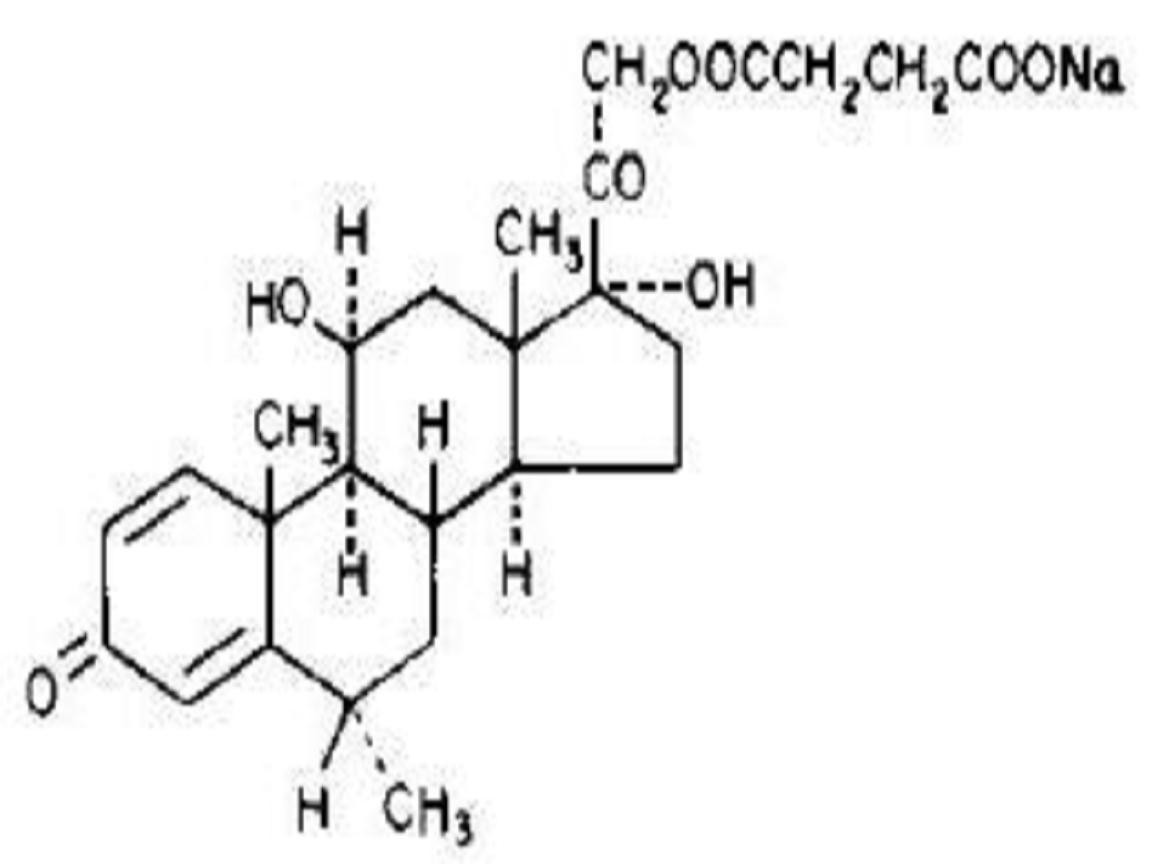
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
Allergic states
Dermatologic diseases
Endocrine disorders
Gastrointestinal diseases
Hematologic disorders
Miscellaneous
Neoplastic diseases
Nervous System
Ophthalmic diseases
Renal diseases
Respiratory diseases
Rheumatic disorders
SOLU-MEDROL CONTRAINDICATIONS
-
● in systemic fungal infections and patients with known hypersensitivity to the product and its constituents.
-
● for intrathecal administration. Reports of severe medical events have been associated with this route of administration.
-
● Intramuscular corticosteroid preparations are contraindicated for idiopathic thrombocytopenic purpura.
WARNINGSPRECAUTIONS, Pediatric Use
WARNINGS
General
Formulations with preservative (seeDESCRIPTION) contain benzyl alcohol, which is potentially toxic when administered locally to neural tissue. PRECAUTIONS, Pediatric Use
ADVERSE REACTIONS
Cardio-renal
Endocrine
Infections
General
Fungal infections
CONTRAINDICATIONSPRECAUTIONS, Drug Interactions, Amphotericin B injection and potassium-depleting agents
Special pathogens
Tuberculosis
Vaccination
Viral infections
Neurologic
ADVERSE REACTIONS, GastrointestinalNeurologic/Psychiatric
Ophthalmic
PRECAUTIONS
GeneralCardio-renal
Endocrine
Gastrointestinal
Musculoskeletal
Neurologic-psychiatric
DOSAGE AND ADMINISTRATION
Ophthalmic
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
AminoglutethimideAmphotericin B injection and potassium-depleting agents
Antibiotics
Drug Interactions, Hepatic Enzyme Inhibitors
Anticholinesterases
Anticoagulants, oral
Antidiabetics
Antitubercular drugs
Cholestyramine
Cyclosporine
Digitalis glycosides
Estrogens, including oral contraceptives
Hepatic Enzyme Inducers (e.g., barbiturates, phenytoin, carbamazepine, rifampin)
Hepatic Enzyme Inhibitors (e.g., ketoconazole, macrolide antibiotics such as erythromycin and troleandomycin)
Ketoconazole
Nonsteroidal anti-inflammatory agents (NSAIDs)
Skin tests
Vaccines
WARNINGS, Infections, Vaccination
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic effectsPregnancy Category C
NURSING MOTHERS
PEDIATRIC USE
DESCRIPTIONADVERSE REACTIONS
GERIATRIC USE
SOLU-MEDROL ADVERSE REACTIONS
WARNINGS
WARNINGS, Neurologic
WARNINGS
OVERDOSAGE
DOSAGE & ADMINISTRATION
NOTE: Some of the SOLU-MEDROL formulations contain benzyl alcohol (seeDESCRIPTION,WARNINGSandPRECAUTIONS, Pediatric Use)Because of possible physical incompatibilities, SOLU-MEDROL should not be diluted or mixed with other solutions.
DESCRIPTION
(greater than 0.5 gram administered over a period of less than 10 minutes).administered intravenously over at least 30 minutes.
PRECAUTIONS, Neurologic-psychiatric
DIRECTIONS FOR USING THE ACT-O-VIAL SYSTEM
STORAGE CONDITIONS
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Solu-MedrolMethylprednisolone Sodium Succinate POWDER, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!