Somnote
Breckenridge Pharmaceutical, Inc.
Somnote (Chloral Hydrate Capsules, USP 500 mg) CIV
FULL PRESCRIBING INFORMATION: CONTENTS*
- SOMNOTE DESCRIPTION
- CLINICAL PHARMACOLOGY
- SOMNOTE INDICATIONS AND USAGE
- SOMNOTE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- SOMNOTE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- SOMNOTE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 50 Capsule Bottle
FULL PRESCRIBING INFORMATION
SOMNOTE DESCRIPTION
Each capsule for oral administration contains:
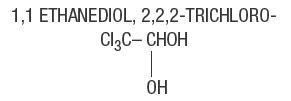
Chloral Hydrate, USP. . . . . . 500 mg
Warning: May be habit forming
Chloral Hydrate acts as a sedative and hypnotic.
INACTIVE INGREDIENTS
Macrogol 400, Gelatin, Glycerin, Sorbitol, Sorbitane, Mannitol, Titanium Dioxide, Iron Oxide.
CLINICAL PHARMACOLOGY
Chloral Hydrate is the oldest member of the hypnotic group of drugs. The action of Chloral Hydrate is confined to the cerebral hemispheres. Chloral Hydrate is detoxified in the liver and subsequently eliminated by the kidney.
SOMNOTE INDICATIONS AND USAGE
Chloral Hydrate is used primarily as a hypnotic in the management of simple insomnia. It is effective as a hypnotic only for short term use. Chloral Hydrate has been found to lose much of its effectiveness for both inducing and maintaining sleep by the end of a 2 week period of administration. Chloral Hydrate may also be used as a routine sedative. It has been used preoperatively or prior to electroencephalographic evaluation to allay anxiety and produce sedation and/or sleep. Chloral Hydrate, alone or in conjunction with Paraldehyde, may be effective in suppressing and/or reducing alcohol withdrawal symptoms. It may also be effective in reducing anxiety associated with withdrawal of other drugs such as narcotics or barbiturates.
SOMNOTE CONTRAINDICATIONS
Chloral Hydrate is contraindicated in patients with marked hepatic or renal impairment and in patients who have previously demonstrated hypersensitivity or an idiosyncratic reaction to the drug.
WARNINGS
Anaphylaxis (severe allergic reaction) and angioedema (severe facial swelling), which can occur as early as the first time the product is taken. Complex sleep-related behaviors which may include sleep-driving, making phone calls, and preparing and eating food (while asleep). Chloral Hydrate may be habit-forming. Chloral Hydrate may increase the rate of metabolism of concomitantly administered Warfarin anticoagulants, thus reducing their effectiveness. Upon withdrawal of Chloral Hydrate, the rate of metabolism of the anticoagulant drug will decrease and its plasma level will rise, with the possibility of a sudden increase of anticoagulant effects (i.e., development of bleeding tendency and hemorrhage). The prothrombin time of patients on anticoagulant therapy normally must be followed continually; however, when Chloral Hydrate is added to or subtracted from the therapeutic regimen, or when changes in dosage of Chloral Hydrate are contemplated, the effect of the sedative on prothrombin time deserves special attention.
Use of this product is not recommended if you are pregnant, or planning to become pregnant or are currently breast-feeding. Please contact your physician, or health-care provider before using or continuing use. (see PRECAUTIONS, Pregnancy )
PRECAUTIONS
General
Chloral Hydrate should be used cautiously in patients who are mentally depressed, have suicidal tendencies or a history of drug abuse, or whose history indicates they may increase dosage on their own initiative. Oral administration of Chloral Hydrate should be avoided in patients with esophagitis, gastritis, or gastric or duodenal ulcers. Large doses of Chloral Hydrate should not be used in patients with severe cardiac disease. Gastritis, skin eruptions, or parenchymatous renal damage may develop following prolonged administration of Chloral Hydrate. Rarely prolonged use of Chloral Hydrate may produce tolerance and physical and/or psychological dependence. Following chronic administration, Chloral Hydrate should be withdrawn slowly to avoid the possibility of precipitating withdrawal symptoms. Chloral Hydrate has been reported to precipitate attacks of acute intermittent porphyria and should be used with caution in susceptible patients.
Information for Patients
The patient should be warned that Chloral Hydrate may impair ability to perform hazardous activities requiring mental alertness or physical coordination such as operating machinery or driving a motor vehicle. In addition, the patient should be cautioned against taking other depressant drugs, including alcohol, while using Choral Hydrate. Chloral Hydrate capsules should be taken with a full glass of water or liquid.
Drug Interactions
Additive central nervous depression may occur when Chloral Hydrate is administered concomitantly with other central nervous system depressants such as paraldehyde, barbiturates or alcohol. In addition, patients receiving Chloral Hydrate may develop a vasodilation reaction characterized by tachycardia, palpitations, facial flushing and dysphoria after ingesting alcohol. If Chloral Hydrate is used concomitantly with other depressant drugs including alcohol, caution should be used to avoid overdosage. A reaction characterized by diaphoresis, flushes, variable blood pressure including hypertension, and uneasiness has been reported on some patients with acute myocardial infarction and congestive heart failure who received Furosemide (Lasix®) intravenously within 24 hours after administration of an oral hypnotic dose of Chloral Hydrate. Therefore, it may be preferable to use an alternate hypnotic drug, e.g., a benzodiazepine, in patients who require intravenously administered Furosemide. Although some investigators believe that the clinical significance of the interaction between Chloral Hydrate and Warfarin anticoagulants is negligible, some clinical studies have shown that concurrent administration of Chloral Hydrate and Warfarin may result in a transient potentiation of Warfarin-induced hypoprothrombinemia. Apparently the trichloroacetic acid metabolite of Chloral Hydrate displaces Warfarin from its binding sites on plasma albumin resulting in a transient increase in free plasma Warfarin. Chloral Hydrate should be used with caution in patients receiving Warfarin or other oral anticoagulants and it is preferable to use an alternative hypnotic drug, e.g., a Benzodiazepine, which does not alter the anticoagulant response in patients receiving anticoagulants. If Chloral Hydrate is added to the therapeutic regimen of a patient maintained on an oral anticoagulant, reduction in anticoagulant dosage may be required in order to prevent excessive hypoprothrombinemia, conversely, the discontinuation of Chloral Hydrate in patients also taking oral anticoagulants may require increased dosage to maintain adequate anticoagulation.
Drug/Laboratory Test Interactions
Chloral Hydrate may produce false-positive results for urine glucose determinations utilizing cupric sulfate as Benedict's Solution. Apparently, the drug does not interfere with urine glucose tests utilizing cupric sulfate tablets (Clinitest®) or glucose oxidase such as Clinistix® or Tes-Tape®. Chloral Hydrate may interfere with fluorometric tests for urine catecholamines, and it has been recommended that the drug not be administered for 48 hours preceding the test. Chloral Hydrate administration may also interfere with the Reddy, Jenkins, Thorn procedure for determining urinary 17-hydroxycorticosteroids.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with Chloral Hydrate. It is also not known whether Chloral Hydrate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. This product should be given to a pregnant woman only if the potential benefit justifies potential risk to the fetus. Chloral Hydrate has been shown to cross the placenta and may be found in the amniotic fluid and fetal blood.
Nursing Mothers
Chloral Hydrate is excreted in human milk. Because of the potential for adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug.
Pediatric Use
Safety and effectiveness in the pediatric population have not been established.
Use in the elderly and/or debilitated patients
Impaired motor and/or cognitive performance after repeated exposure or unusual sensitivity to sedative/hypnotic drugs is a concern in the treatment of elderly and/or debilitated patients. These patients should be closely monitored.
SOMNOTE ADVERSE REACTIONS
Gastric irritation manifested by nausea, vomiting, and diarrhea is the most frequent side effect of oral Chloral Hydrate administration. This effect may be minimized by administering the capsule with a full glass of water or other liquid. There is no evidence in the published literature to support the claim that the complex, chloral betaine, causes less gastric irritation than does Chloral Hydrate. Residual sedation or "hangover" occurs infrequently following usual hypnotic doses. Cutaneous reactions to Chloral Hydrate are not common but have included scarlatiniform or erythematous rash, urticaria, angioedema, purpura, eczema, bullous lesions, and erythema multiforme. Sometimes these cutaneous reactions have been accompanied by fever; ataxia and dizziness have also occurred. Rarely, a somnambulistic reaction characterized by disorientation and incoherence has been reported.
DRUG ABUSE AND DEPENDENCE
Controlled Substance
Chloral Hydrate is a Schedule IV drug.
Abuse
Chloral hydrate may be habit forming.
Dependence
Symptoms of Chloral Hydrate dependence are similar to those of chronic alcoholism. Sudden withdrawal of the drug from physically dependent persons may cause delirium tremens and hallucinations. For this reason, Chloral Hydrate should be withdrawn slowly.
OVERDOSAGE
Chloral Hydrate overdosage produces symptoms which are similar to those of barbiturate overdosage and may include coma, hypotension, hypothermia, respiratory depression and cardiac arrhythmias. Miosis, vomiting, areflexia, and muscle flaccidity may also occur. Esophageal stricture, gastric necrosis and perforation, and gastrointestinal hemorrhage have also been reported. Hepatic and Renal function may be impaired and may result in transient jaundice and/or albuminuria. Death may result from respiratory failure or hypertension. Ingestion of 4 grams of Chloral Hydrate has caused death, although some patients have survived the ingestion of as much as 30 grams of the drug. Treatment of Chloral Hydrate intoxication consists of general supportive therapy including maintenance of an adequate airway, assisted respiration, oxygen administration, and maintaining body temperature and circulation. Gastric lavage may be done following oral overdosage if an endotracheal tube with cuff inflated is in place to prevent aspiration of vomitus. Peritoneal dialysis or hemodialysis may be beneficial.
SOMNOTE DOSAGE AND ADMINISTRATION
The usual hypnotic dose of Chloral Hydrate for adults is 500 mg to 1 gram 15 to 30 minutes before retiring. The usual sedative dosage is 250 mg 3 times daily after meals. When Choral Hydrate is administered in the management of alcohol withdrawal symptoms, the usual dosage is 500 mg to 1 gram repeated at 6-hour intervals if needed. Generally, single doses or daily dosage for adults should not exceed 2 grams.
HOW SUPPLIED
Somnote® Capsules are supplied as gray, oval-shaped softgel capsules, imprinted "B-080". Available in unit dose packaging 50 capsules (5 × 10), NDC 51991-080-51, and bottles containing 50 capsules, NDC 51991-080-52.
Store at 25°C (77°F); excursions permitted to 15°-30°C(59°-86°F). See USP Controlled Room Temperature. Protect from freezing.
Dispense in a tight, light-resistant container with a child-resistant closure as defined in the USP/NF.
WARNING: Keep this and all medications out of the reach of children. In case of accidental overdose, seek professional assistance or contact a poison control center immediately.
Manufactured by:
G. Pohl Boskamp GmbH & Co.
Hohenlockstedt, Germany
Distributed By:
Breckenridge Pharmaceutical, Inc.
Boca Raton, FL 33487
REV. 5/09
Rx Only
PRINCIPAL DISPLAY PANEL - 50 Capsule Bottle
Breckenridge
Pharmaceutical, Inc.
NDC 51991-080-52
Somnote®
(Chloral Hydrate Capsules, USP)
500 mg
Rx Only
50 Capsules
New
Appearance

SomnoteChloral Hydrate CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||