Spiriva
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SPIRIVA HandiHaler safely and effectively. See full prescribing information for SPIRIVA HandiHaler. SPIRIVA HandiHaler (tiotropium bromide inhalation powder) Capsules for Respiratory Inhalation DO NOT Swallow SPIRIVA Capsules FOR ORAL INHALATION ONLY with the HandiHaler Device Initial U.S. Approval: 2004RECENT MAJOR CHANGES Indications and Usage (1) 12/2009 Dosage and Administration (2) 12/2009 Contraindications (4) 12/2009 Warnings and Precautions, Immediate Hypersensitivity Reactions (5.2) 12/2009 Worsening of Narrow-Angle Glaucoma (5.4) 12/2009 Worsening of Urinary Retention (5.5) 12/2009 Renal Impairment (5.6) 12/2009 INDICATIONS AND USAGESPIRIVA HandiHaler is an anticholinergic indicated for the long-term, once-daily, maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD), and for reducing COPD exacerbations (1) DOSAGE AND ADMINISTRATIONDO NOT swallow SPIRIVA capsules (2) For Use with the HandiHaler Device ONLY (2) For Oral Inhalation ONLY (2) Two inhalations of the powder contents of a single SPIRIVA capsule (18 mcg) once daily (2) DOSAGE FORMS AND STRENGTHSSPIRIVA capsules for oral inhalation: 18 mcg tiotropium powder, for use with HandiHaler device (3) CONTRAINDICATIONS Hypersensitivity to ipratropium or tiotropium (4) WARNINGS AND PRECAUTIONS Not for acute use: Not for use as a rescue medication (5.1) Immediate hypersensitivity reactions: Discontinue SPIRIVA HandiHaler at once and consider alternatives if immediate hypersensitivity reactions, including angioedema, occur. Use with caution in patients with severe hypersensitivity to milk proteins. (5.2) Paradoxical bronchospasm: Discontinue SPIRIVA HandiHaler and consider other treatments if paradoxical bronchospasm occurs (5.3) Worsening of narrow-angle glaucoma may occur. Use with caution in patients with narrow-angle glaucoma and instruct patients to consult a physician immediately if this occurs. (5.4) Worsening of urinary retention may occur. Use with caution in patients with prostatic hyperplasia or bladder-neck obstruction and instruct patients to consult a physician immediately if this occurs. (5.5) Side Effects The most common adverse reactions (>5% incidence in the 1-year placebo-controlled trials) were upper respiratory tract infection, dry mouth, sinusitis, pharyngitis, non-specific chest pain, urinary tract infection, dyspepsia, and rhinitis (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at (800) 542-6257 or (800) 459-9906 TTY, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSNot recommended for use with other anticholinergics since this has not been studied (7.2) USE IN SPECIFIC POPULATIONSPatients with moderate to severe renal impairment should be monitored closely for potential anticholinergic side effects (2, 8.6)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 SPIRIVA INDICATIONS AND USAGE
- 2 SPIRIVA DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 SPIRIVA CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 SPIRIVA ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 SPIRIVA DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
SPIRIVA HandiHaler (tiotropium bromide inhalation powder) is indicated for the long-term, once-daily, maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. SPIRIVA HandiHaler is indicated to reduce exacerbations in COPD patients.
2 DOSAGE AND ADMINISTRATION
DO NOT SWALLOW SPIRIVA CAPSULES
FOR USE WITH HANDIHALER DEVICE ONLY
FOR ORAL INHALATION ONLY
SPIRIVA capsules must not be swallowed as the intended effects on the lungs will not be obtained. The contents of the SPIRIVA capsules are only for oral inhalation and should only be used with the HandiHaler device [ see Overdosage (10) ].
The recommended dose of SPIRIVA HandiHaler is two inhalations of the powder contents of one SPIRIVA capsule, once-daily, with the HandiHaler device [ see Patient Counseling Information (17.6) ].
For administration of SPIRIVA HandiHaler, a SPIRIVA capsule is placed into the center chamber of the HandiHaler device. The SPIRIVA capsule is pierced by pressing and releasing the green piercing button on the side of the HandiHaler device. The tiotropium formulation is dispersed into the air stream when the patient inhales through the mouthpiece [ see Patient Counseling Information (17.6) ].
No dosage adjustment is required for geriatric, hepatically-impaired, or renally-impaired patients. However, patients with moderate to severe renal impairment given SPIRIVA HandiHaler should be monitored closely for anticholinergic effects [ see Warnings and Precautions (5.6), Use in Specific Populations (8.5, 8.6, 8.7), and Clinical Pharmacology (12.3) ].
3 DOSAGE FORMS AND STRENGTHS
SPIRIVA HandiHaler consists of SPIRIVA capsules and a HandiHaler device. SPIRIVA capsules contain 18 mcg dry powder formulation of tiotropium in a light green, hard gelatin capsule with TI 01 printed on one side and Boehringer Ingelheim company logo on the other side. Supplied with a HandiHaler device.
4 CONTRAINDICATIONS
SPIRIVA HandiHaler is contraindicated in patients with a hypersensitivity to ipratropium or tiotropium. In clinical trials and postmarketing experience with SPIRIVA HandiHaler, immediate hypersensitivity reactions, including angioedema (including swelling of the lips, tongue, or throat), itching, or rash have been reported.
5 WARNINGS AND PRECAUTIONS
5.1 Not for Acute Use
SPIRIVA HandiHaler is intended as a once-daily maintenance treatment for COPD and is not indicated for the initial treatment of acute episodes of bronchospasm (i.e., rescue therapy).
5.2 Immediate Hypersensitivity Reactions
Immediate hypersensitivity reactions, including angioedema (including swelling of the lips, tongue, or throat), itching, or rash may occur after administration of SPIRIVA HandiHaler. If such a reaction occurs, therapy with SPIRIVA HandiHaler should be stopped at once and alternative treatments should be considered. Given the similar structural formula of atropine to tiotropium, patients with a history of hypersensitivity reactions to atropine should be closely monitored for similar hypersensitivity reactions to SPIRIVA HandiHaler. In addition, SPIRIVA HandiHaler should be used with caution in patients with severe hypersensitivity to milk proteins.
5.3 Paradoxical Bronchospasm
Inhaled medicines, including SPIRIVA HandiHaler, may cause paradoxical bronchospasm. If this occurs, treatment with SPIRIVA HandiHaler should be stopped and other treatments considered.
5.4 Worsening of Narrow-Angle Glaucoma
SPIRIVA HandiHaler should be used with caution in patients with narrow-angle glaucoma. Prescribers and patients should be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately should any of these signs or symptoms develop.
5.5 Worsening of Urinary Retention
SPIRIVA HandiHaler should be used with caution in patients with urinary retention. Prescribers and patients should be alert for signs and symptoms of prostatic hyperplasia or bladder-neck obstruction (e.g., difficulty passing urine, painful urination). Instruct patients to consult a physician immediately should any of these signs or symptoms develop.
5.6 Renal Impairment
As a predominantly renally excreted drug, patients with moderate to severe renal impairment (creatinine clearance of ≤50 mL/min) treated with SPIRIVA HandiHaler should be monitored closely for anticholinergic side effects [ see Clinical Pharmacology (12.3) ].
6 ADVERSE REACTIONS
The following adverse reactions are described, or described in greater detail, in other sections:
- Immediate hypersensitivity reactions [ see Warnings and Precautions (5.2) ]
- Paradoxical bronchospasm [ see Warnings and Precautions (5.3) ]
- Worsening of narrow-angle glaucoma [ see Warnings and Precautions (5.4) ]
- Worsening of urinary retention [ see Warnings and Precautions (5.5) ]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6-Month to 1-Year Trials
The data described below reflect exposure to SPIRIVA HandiHaler in 2663 patients. SPIRIVA HandiHaler was studied in two 1-year placebo-controlled trials, two 1-year active-controlled trials, and two 6-month placebo-controlled trials in patients with COPD. In these trials, 1308 patients were treated with SPIRIVA HandiHaler at the recommended dose of 18 mcg once a day. The population had an age ranging from 39 to 87 years with 65% to 85% males, 95% Caucasian, and had COPD with a mean pre-bronchodilator forced expiratory volume in one second (FEV1) percent predicted of 39% to 43%. Patients with narrow-angle glaucoma, or symptomatic prostatic hypertrophy or bladder outlet obstruction were excluded from these trials. An additional 6-month trial conducted in a Veteran's Affairs setting is not included in this safety database because only serious adverse events were collected.
The most commonly reported adverse drug reaction was dry mouth. Dry mouth was usually mild and often resolved during continued treatment. Other reactions reported in individual patients and consistent with possible anticholinergic effects included constipation, tachycardia, blurred vision, glaucoma (new onset or worsening), dysuria, and urinary retention.
Four multicenter, 1-year, placebo-controlled and active-controlled trials evaluated SPIRIVA HandiHaler in patients with COPD. Table 1 shows all adverse reactions that occurred with a frequency of ≥3% in the SPIRIVA HandiHaler group in the 1-year placebo-controlled trials where the rates in the SPIRIVA HandiHaler group exceeded placebo by ≥1%. The frequency of corresponding reactions in the ipratropium-controlled trials is included for comparison.
Table 1 Adverse Reactions (% Patients) in One-Year COPD Clinical Trials
| Body System (Event) | Placebo-Controlled Trials | Ipratropium-Controlled Trials | ||
|---|---|---|---|---|
| SPIRIVA (n = 550) |
Placebo (n = 371) |
SPIRIVA (n = 356) |
Ipratropium (n = 179) |
|
|
Body as a Whole
Chest Pain (non-specific) |
7 | 5 | 5 | 2 |
| Edema, Dependent | 5 | 4 | 3 | 5 |
|
Gastrointestinal System Disorders
Dry Mouth |
16 | 3 | 12 | 6 |
| Dyspepsia | 6 | 5 | 1 | 1 |
| Abdominal Pain | 5 | 3 | 6 | 6 |
| Constipation | 4 | 2 | 1 | 1 |
| Vomiting | 4 | 2 | 1 | 2 |
|
Musculoskeletal System
Myalgia |
4 | 3 | 4 | 3 |
|
Resistance Mechanism Disorders
Infection |
4 | 3 | 1 | 3 |
| Moniliasis | 4 | 2 | 3 | 2 |
|
Respiratory System (Upper)
Upper Respiratory Tract Infection |
41 | 37 | 43 | 35 |
| Sinusitis | 11 | 9 | 3 | 2 |
| Pharyngitis | 9 | 7 | 7 | 3 |
| Rhinitis | 6 | 5 | 3 | 2 |
| Epistaxis | 4 | 2 | 1 | 1 |
|
Skin and Appendage Disorders
Rash |
4 | 2 | 2 | 2 |
|
Urinary System
Urinary Tract Infection |
7 | 5 | 4 | 2 |
Arthritis, coughing, and influenza-like symptoms occurred at a rate of ≥3% in the SPIRIVA HandiHaler treatment group, but were <1% in excess of the placebo group.
Other reactions that occurred in the SPIRIVA HandiHaler group at a frequency of 1% to 3% in the placebo-controlled trials where the rates exceeded that in the placebo group include: Body as a Whole: allergic reaction, leg pain; Central and Peripheral Nervous System: dysphonia, paresthesia; Gastrointestinal System Disorders: gastrointestinal disorder not otherwise specified (NOS), gastroesophageal reflux, stomatitis (including ulcerative stomatitis); Metabolic and Nutritional Disorders: hypercholesterolemia, hyperglycemia; Musculoskeletal System Disorders: skeletal pain; Cardiac Events: angina pectoris (including aggravated angina pectoris); Psychiatric Disorder: depression; Infections: herpes zoster; Respiratory System Disorder (Upper): laryngitis; Vision Disorder: cataract. In addition, among the adverse reactions observed in the clinical trials with an incidence of <1% were atrial fibrillation, supraventricular tachycardia, angioedema, and urinary retention.
In the 1-year trials, the incidence of dry mouth, constipation, and urinary tract infection increased with age [ see Use in Specific Populations (8.5) ].
Two multicenter, 6-month, controlled studies evaluated SPIRIVA HandiHaler in patients with COPD. The adverse reactions and the incidence rates were similar to those seen in the 1-year controlled trials.
4-Year Trial
The data described below reflect exposure to SPIRIVA
HandiHaler in 5992 COPD patients in a 4-year placebo-controlled trial. In this
trial, 2986 patients were treated with SPIRIVA HandiHaler at the recommended
dose of 18 mcg once a day. The population had an age range from 40 to 88
years, was 75% male, 90% Caucasian, and had COPD with a mean pre-bronchodilator
FEV1 percent predicted of 40%. Patients with narrow-angle
glaucoma, or symptomatic prostatic hypertrophy or bladder outlet obstruction
were excluded from these trials. When the adverse reactions were analyzed with
a frequency of ≥3% in the SPIRIVA HandiHaler group where the rates in the
SPIRIVA HandiHaler group exceeded placebo by ≥1%, adverse reactions included (SPIRIVA HandiHaler, placebo): pharyngitis (12.5%, 10.8%), sinusitis (6.5%, 5.3%), headache (5.7%, 4.5%), constipation (5.1%, 3.7%), dry mouth (5.1%, 2.7%), depression (4.4%, 3.3%), insomnia (4.4%, 3.0%), and arthralgia (4.2%, 3.1%).
Additional
Adverse Reactions
Other adverse reactions not previously listed that were reported more frequently in
COPD patients treated with SPIRIVA HandiHaler than placebo include:
dehydration, skin ulcer, stomatitis, gingivitis, oropharyngeal candidiasis, dry
skin, skin infection, and joint swelling.
6.2 Postmarketing Experience
Adverse reactions have been identified during worldwide post-approval use of SPIRIVA HandiHaler. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These adverse reactions are: application site irritation (glossitis, mouth ulceration, and pharyngolaryngeal pain), dizziness, dysphagia, hoarseness, intestinal obstruction including ileus paralytic, intraocular pressure increased, oral candidiasis, palpitations, pruritus, tachycardia, throat irritation, and urticaria.
7 DRUG INTERACTIONS
7.1 Sympathomimetics, Methylxanthines, Steroids
SPIRIVA HandiHaler has been used concomitantly with short-acting and long-acting sympathomimetic (beta-agonists) bronchodilators, methylxanthines, and oral and inhaled steroids without increases in adverse drug reactions.
7.2 Anticholinergics
The co-administration of SPIRIVA HandiHaler with other anticholinergic-containing drugs (e.g., ipratropium) has not been studied and is therefore not recommended.
7.3 Cimetidine, Ranitidine
No clinically significant interaction occurred between tiotropium and cimetidine or ranitidine [ see Clinical Pharmacology (12.3) ].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic
Effects, Pregnancy Category C.
There are no adequate and well-controlled studies in pregnant women. SPIRIVA
HandiHaler should be used during pregnancy only if the potential benefit
justifies the potential risk to the fetus.
No evidence of structural alterations was observed in rats and rabbits at inhalation tiotropium doses of up to approximately 660 and 6 times the recommended human daily inhalation dose (RHDID) on a mg/m2 basis, respectively. However, in rats, tiotropium caused fetal resorption, litter loss, decreases in the number of live pups at birth and the mean pup weights, and a delay in pup sexual maturation at inhalation tiotropium doses of approximately 35 times the RHDID on a mg/m2 basis. In rabbits, tiotropium caused an increase in post-implantation loss at an inhalation dose of approximately 360 times the RHDID on a mg/m2 basis. Such effects were not observed at inhalation doses of approximately 4 and 80 times the RHDID on a mg/m2 basis, respectively. These dose multiples may be over-estimated due to difficulties in measuring deposited doses in animal inhalation studies.
8.2 Labor and Delivery
The safety and effectiveness of SPIRIVA HandiHaler has not been studied during labor and delivery.
8.3 Nursing Mothers
Clinical data from nursing women exposed to tiotropium are not available. Based on lactating rodent studies, tiotropium is excreted into breast milk. It is not known whether tiotropium is excreted in human milk, but because many drugs are excreted in human milk and given these findings in rats, caution should be exercised if SPIRIVA HandiHaler is administered to a nursing woman.
8.4 Pediatric Use
SPIRIVA HandiHaler is approved for use in the maintenance treatment of bronchospasm associated with COPD and for the reduction of COPD exacerbations. COPD does not normally occur in children. The safety and effectiveness of SPIRIVA HandiHaler in pediatric patients have not been established.
8.5 Geriatric Use
Of the total number of patients who received SPIRIVA HandiHaler in the 1-year clinical trials, 426 were <65 years, 375 were 65 to 74 years, and 105 were ≥75 years of age. Within each age subgroup, there were no differences between the proportion of patients with adverse events in the SPIRIVA HandiHaler and the comparator groups for most events. Dry mouth increased with age in the SPIRIVA HandiHaler group (differences from placebo were 9.0%, 17.1%, and 16.2% in the aforementioned age subgroups). A higher frequency of constipation and urinary tract infections with increasing age was observed in the SPIRIVA HandiHaler group in the placebo-controlled studies. The differences from placebo for constipation were 0%, 1.8%, and 7.8% for each of the age groups. The differences from placebo for urinary tract infections were –0.6%, 4.6%, and 4.5%. No overall differences in effectiveness were observed among these groups. Based on available data, no adjustment of SPIRIVA HandiHaler dosage in geriatric patients is warranted [ see Clinical Pharmacology (12.3) ].
8.6 Renal Impairment
Patients with moderate to severe renal impairment (creatinine clearance of ≤50 mL/min) treated with SPIRIVA HandiHaler should be monitored closely for anticholinergic side effects [ see Dosage and Administration (2), Warnings and Precautions (5.4), and Clinical Pharmacology (12.3) ].
8.7 Hepatic Impairment
The effects of hepatic impairment on the pharmacokinetics of tiotropium were not studied.
10 OVERDOSAGE
High doses of tiotropium may lead to anticholinergic signs and symptoms. However, there were no systemic anticholinergic adverse effects following a single inhaled dose of up to 282 mcg tiotropium in 6 healthy volunteers. In a study of 12 healthy volunteers, bilateral conjunctivitis and dry mouth were seen following repeated once-daily inhalation of 141 mcg of tiotropium.
Accidental Ingestion
Acute intoxication
by inadvertent oral ingestion of SPIRIVA capsules is unlikely since it is not
well-absorbed systemically.
A case of overdose has been reported from postmarketing experience. A female patient was reported to have inhaled 30 capsules over a 2.5 day period, and developed altered mental status, tremors, abdominal pain, and severe constipation. The patient was hospitalized, SPIRIVA HandiHaler was discontinued, and the constipation was treated with an enema. The patient recovered and was discharged on the same day.
No mortality was observed at inhalation tiotropium doses up to 32.4 mg/kg in mice, 267.7 mg/kg in rats, and 0.6 mg/kg in dogs. These doses correspond to 7300, 120,000, and 850 times the recommended human daily inhalation dose on a mg/m2 basis, respectively. These dose multiples may be over-estimated due to difficulties in measuring deposited doses in animal inhalation studies.
11 DESCRIPTION
SPIRIVA HandiHaler consists of a capsule dosage form containing a dry powder formulation of tiotropium intended for oral inhalation only with the HandiHaler device.
Each light green, hard gelatin SPIRIVA capsule contains 18 mcg tiotropium (equivalent to 22.5 mcg tiotropium bromide monohydrate) blended with lactose monohydrate (which may contain milk proteins) as the carrier.
The dry powder formulation within the SPIRIVA capsule is intended for oral inhalation only.
The active component of SPIRIVA HandiHaler is tiotropium. The drug substance, tiotropium bromide monohydrate, is an anticholinergic with specificity for muscarinic receptors. It is chemically described as (1α, 2ß, 4ß, 5α, 7ß)-7-[(Hydroxydi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonane bromide monohydrate. It is a synthetic, non-chiral, quaternary ammonium compound. Tiotropium bromide is a white or yellowish white powder. It is sparingly soluble in water and soluble in methanol.
The structural formula is:
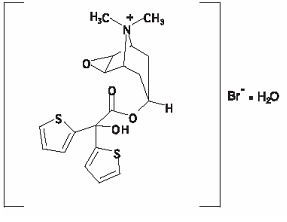
Tiotropium bromide (monohydrate) has a molecular mass of 490.4 and a molecular formula of C19H22NO4S2Br • H2O.
The HandiHaler device is an inhalation device used to inhale the dry powder contained in the SPIRIVA capsule. The dry powder is delivered from the HandiHaler device at flow rates as low as 20 L/min. Under standardized in vitro testing, the HandiHaler device delivers a mean of 10.4 mcg tiotropium when tested at a flow rate of 39 L/min for 3.1 seconds (2 L total). In a study of 26 adult patients with COPD and severely compromised lung function [mean FEV1 1.02 L (range 0.45 to 2.24 L); 37.6% of predicted (range 16% to 65%)], the median peak inspiratory flow (PIF) through the HandiHaler device was 30.0 L/min (range 20.4 to 45.6 L/min). The amount of drug delivered to the lungs will vary depending on patient factors such as inspiratory flow and peak inspiratory flow through the HandiHaler device, which may vary from patient to patient, and may vary with the exposure time of the SPIRIVA capsule outside the blister pack.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tiotropium is a long-acting, antimuscarinic agent, which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors, M1 to M5. In the airways, it exhibits pharmacological effects through inhibition of M3-receptors at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivo studies, prevention of methacholine-induced bronchoconstriction effects was dose-dependent and lasted longer than 24 hours. The bronchodilation following inhalation of tiotropium is predominantly a site-specific effect.
12.2 Pharmacodynamics
Cardiovascular
Effects
In a multicenter, randomized, double-blind trial that enrolled 198 patients with
COPD, the number of subjects with changes from baseline-corrected QT interval
of 30 to 60 msec was higher in the SPIRIVA HandiHaler group as compared with
placebo. This difference was apparent using both the Bazett (QTcB) [20 (20%)
patients vs 12 (12%) patients] and Fredericia (QTcF) [16 (16%) patients vs 1 (1%) patient] corrections of QT for heart rate. No patients in either group had
either QTcB or QTcF of >500 msec. Other clinical studies with SPIRIVA
HandiHaler did not detect an effect of the drug on QTc intervals.
The effect of SPIRIVA HandiHaler on QT interval was also evaluated in a randomized, placebo- and positive-controlled crossover study in 53 healthy volunteers. Subjects received SPIRIVA HandiHaler 18 mcg, 54 mcg (3 times the recommended dose), or placebo for 12 days. ECG assessments were performed at baseline and throughout the dosing interval following the first and last dose of study medication. Relative to placebo, the maximum mean change from baseline in study-specific QTc interval was 3.2 msec and 0.8 msec for SPIRIVA HandiHaler 18 mcg and 54 mcg, respectively. No subject showed a new onset of QTc >500 msec or QTc changes from baseline of ≥60 msec.
12.3 Pharmacokinetics
Tiotropium is administered by dry powder inhalation. In common with other inhaled drugs, the majority of the delivered dose is deposited in the gastrointestinal tract and, to a lesser extent, in the lung, the intended organ. Many of the pharmacokinetic data described below were obtained with higher doses than recommended for therapy.
Absorption
Following dry powder inhalation by young healthy volunteers, the absolute bioavailability
of 19.5% suggests that the fraction reaching the lung is highly bioavailable.
It is expected from the chemical structure of the compound (quaternary ammonium
compound) that tiotropium is poorly absorbed from the gastrointestinal tract. The
effect of food on tiotropium's bioavailability has not been studied. Oral
solutions of tiotropium have an absolute bioavailability of 2% to 3%.
Maximum tiotropium plasma concentrations were observed 5 minutes after
inhalation.
Distribution
Tiotropium shows a volume of distribution of 32 L/kg indicating that the drug binds
extensively to tissues. The human plasma protein binding for tiotropium is 72%.
At steady state, peak tiotropium plasma levels in COPD patients were 17 to 19
pg/mL when measured 5 minutes after dry powder inhalation of an 18 mcg dose and
decreased in a multi-compartmental manner. Steady-state trough plasma
concentrations were 3 to 4 pg/mL. Local concentrations in the lung
are not known, but the mode of administration suggests substantially higher
concentrations in the lung. Studies in rats have shown that tiotropium does not
readily penetrate the blood-brain barrier.
Metabolism
The extent of metabolism appears to be small. This is evident from a urinary
excretion of 74% of unchanged substance after an intravenous dose to young
healthy volunteers. Tiotropium, an ester, is nonenzymatically cleaved to the
alcohol
N
-methylscopine and dithienylglycolic acid, neither of which
bind to muscarinic receptors.
In vitro experiments with human liver microsomes and human hepatocytes suggest that a fraction of the administered dose (74% of an intravenous dose is excreted unchanged in the urine, leaving 25% for metabolism) is metabolized by cytochrome P450-dependent oxidation and subsequent glutathione conjugation to a variety of Phase II metabolites. This enzymatic pathway can be inhibited by CYP450 2D6 and 3A4 inhibitors, such as quinidine, ketoconazole, and gestodene. Thus, CYP450 2D6 and 3A4 are involved in the metabolic pathway that is responsible for the elimination of a small part of the administered dose. In vitro studies using human liver microsomes showed that tiotropium in supra-therapeutic concentrations did not inhibit CYP450 1A1, 1A2, 2B6, 2C9, 2C19, 2D6, 2E1, or 3A4.
Elimination
The terminal elimination half-life of tiotropium was between 5 and 6 days following
inhalation. Total clearance was 880 mL/min after an intravenous dose in young
healthy volunteers with an inter-individual variability of 22%. Intravenously
administered tiotropium was mainly excreted unchanged in urine (74%). After dry
powder inhalation, urinary excretion was 14% of the dose, the remainder being
mainly non-absorbed drug in the gut which was eliminated via the feces. The
renal clearance of tiotropium exceeds the creatinine clearance, indicating
active secretion into the urine. After chronic once-daily inhalation by COPD
patients, pharmacokinetic steady state was reached after 2 to 3 weeks
with no accumulation thereafter.
Drug Interactions
An interaction study with tiotropium (14.4 mcg intravenous infusion over 15
minutes) and cimetidine 400 mg three times daily or ranitidine 300 mg once
daily was conducted. Concomitant administration of cimetidine with tiotropium
resulted in a 20% increase in the AUC0-4h, a 28% decrease in the
renal clearance of tiotropium and no significant change in the Cmax
and amount excreted in urine over 96 hours. Co-administration of tiotropium
with ranitidine did not affect the pharmacokinetics of tiotropium.
Specific
Populations
Geriatric
Patients
As expected for drugs predominantly excreted renally, advanced age was associated
with a decrease of tiotropium renal clearance (326 mL/min in COPD patients
<58 years to 163 mL/min in COPD patients >70 years), which may be
explained by decreased renal function. Tiotropium excretion in urine after
inhalation decreased from 14% (young healthy volunteers) to about 7% (COPD
patients). Plasma concentrations were numerically increased with advancing age
within COPD patients (43% increase in AUC0-4 after dry powder
inhalation), which was not significant when considered in relation to inter-
and intra-individual variability [
see
Dosage and Administration (2) and
Use in Specific Populations (8.5)
].
Renal
Impairment
Since tiotropium is predominantly renally excreted, renal impairment was associated
with increased plasma drug concentrations and reduced drug clearance after both
intravenous infusion and dry powder inhalation. Mild renal impairment (creatinine
clearance of 50 to 80 mL/min), which is often seen in elderly patients,
increased tiotropium plasma concentrations (39% increase in AUC0-4 after
intravenous infusion). In COPD patients with moderate to severe renal
impairment (creatinine clearance of <50 mL/min), the intravenous
administration of tiotropium resulted in doubling of the plasma concentrations
(82% increase in AUC0-4), which was confirmed by plasma
concentrations after dry powder inhalation. Patients with moderate to severe
renal impairment (creatinine clearance of ≤50 mL/min) treated with
SPIRIVA HandiHaler should be monitored closely for anticholinergic side effects
[
see Dosage and Administration (2),
Warnings and Precautions (5.4),
and Use in Specific Populations (8.6)
].
Hepatic
Impairment
The effects of hepatic impairment on the pharmacokinetics of tiotropium were not
studied.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of tumorigenicity was observed in a 104-week inhalation study in rats at tiotropium doses up to 0.059 mg/kg/day, in an 83-week inhalation study in female mice at doses up to 0.145 mg/kg/day, and in a 101-week inhalation study in male mice at doses up to 0.002 mg/kg/day. These doses correspond to approximately 25, 35, and 0.5 times the recommended human daily inhalation dose (RHDID) on a mg/m2 basis, respectively. These dose multiples may be over-estimated due to difficulties in measuring deposited doses in animal inhalation studies.
Tiotropium bromide demonstrated no evidence of mutagenicity or clastogenicity in the following assays: the bacterial gene mutation assay, the V79 Chinese hamster cell mutagenesis assay, the chromosomal aberration assays in human lymphocytes in vitro and mouse micronucleus formation in vivo , and the unscheduled DNA synthesis in primary rat hepatocytes in vitro assay.
In rats, decreases in the number of corpora lutea and the percentage of implants were noted at inhalation tiotropium doses of 0.078 mg/kg/day or greater (approximately 35 times the RHDID on a mg/m2 basis). No such effects were observed at 0.009 mg/kg/day (approximately 4 times than the RHDID on a mg/m2 basis). The fertility index, however, was not affected at inhalation doses up to 1.689 mg/kg/day (approximately 760 times the RHDID on a mg/m2 basis). These dose multiples may be over-estimated due to difficulties in measuring deposited doses in animal inhalation studies.
13.2 Animal Toxicology and Pharmacology
Reproductive
Toxicology Studies
No evidence of fetal structural alteration was observed in rats and rabbits at
inhalation tiotropium doses of up to 1.471 and 0.007 mg/kg/day, respectively.
These doses correspond to approximately 660 and 6 times the RHDID on a mg/m2 basis, respectively. However, in rats, fetal
resorption, litter loss, decreases in the number of live pups at birth and the
mean pup weights, and a delay in pup sexual maturation were observed at
inhalation tiotropium doses of ≥0.078 mg/kg (approximately 35 times the
RHDID on a mg/m2 basis). In rabbits, an increase in post-implantation
loss was observed at an inhalation dose of 0.4 mg/kg/day (approximately 360
times the RHDID on a mg/m2 basis).
Such effects were not observed at inhalation doses of 0.009 and up to 0.088
mg/kg/day in rats and rabbits, respectively. These doses correspond to
approximately 4 and 80 times the RHDID on a mg/m2 basis, respectively. These dose multiples may be
over-estimated due to difficulties in measuring deposited doses in animal
inhalation studies.
14 CLINICAL STUDIES
The SPIRIVA HandiHaler (tiotropium bromide inhalation powder) clinical development program consisted of six Phase 3 studies in 2663 patients with COPD (1308 receiving SPIRIVA HandiHaler): two 1-year, placebo‑controlled studies, two 6-month, placebo-controlled studies and two 1-year, ipratropium-controlled studies. These studies enrolled patients who had a clinical diagnosis of COPD, were 40 years of age or older, had a history of smoking greater than 10 pack-years, had a forced expiratory volume in one second (FEV1) less than or equal to 60% or 65% of predicted, and a ratio of FEV1/FVC of less than or equal to 0.7.
In these studies, SPIRIVA HandiHaler, administered once-daily in the morning, provided improvement in lung function (FEV1), with peak effect occurring within 3 hours following the first dose.
Two additional trials evaluated exacerbations: a 6-month, randomized, double-blind, placebo-controlled, multicenter clinical trial of 1829 COPD patients in a US Veterans Affairs setting and a 4-year, randomized, double-blind, placebo-controlled, multicenter, clinical trial of 5992 COPD patients. Long-term effects on lung function and other outcomes were also evaluated in the 4-year multicenter trial.
6-Month to 1-Year
Effects on Lung Function
In the 1-year, placebo-controlled trials, the mean improvement in FEV1 at
30 minutes was 0.13 liters (13%) with a peak improvement of 0.24 liters
(24%) relative to baseline after the first dose (Day 1). Further improvements
in FEV1 and forced vital capacity (FVC) were observed with
pharmacodynamic steady state reached by Day 8 with once-daily treatment. The
mean peak improvement in FEV1, relative to baseline, was 0.28 to
0.31 liters (28% to 31%), after 1 week (Day 8) of once-daily treatment. Improvement
of lung function was maintained for 24 hours after a single dose and
consistently maintained over the 1-year treatment period with no evidence of
tolerance.
In the two 6-month, placebo-controlled trials, serial spirometric evaluations were performed throughout daytime hours in Trial A (12 hours) and limited to 3 hours in Trial B. The serial FEV1 values over 12 hours (Trial A) are displayed in Figure 1. These trials further support the improvement in pulmonary function (FEV1) with SPIRIVA HandiHaler, which persisted over the spirometric observational period. Effectiveness was maintained for 24 hours after administration over the 6-month treatment period.
Figure 1 Mean FEV1 Over Time (prior to and after administration of study drug) on Days 1 and 169 for Trial A (a Six-Month Placebo-Controlled Study)*
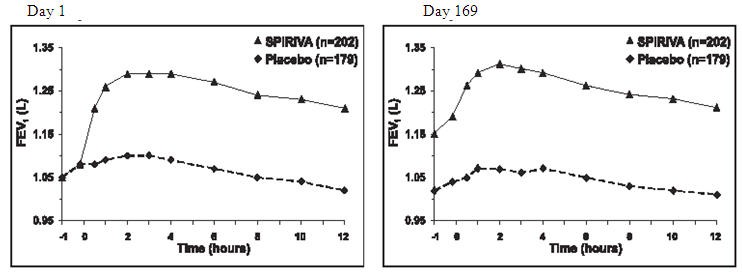
*Means adjusted for center, treatment, and baseline effect. On Day 169, a total of 183 and 149 patients in the SPIRIVA HandiHaler and placebo groups, respectively, completed the trial. The data for the remaining patients were imputed using the last observation or least favorable observation carried forward.
Results of each of the 1-year ipratropium-controlled trials were similar to the results of the 1-year placebo-controlled trials. The results of one of these trials are shown in Figure 2.
Figure 2 Mean FEV1 Over Time (0 to 6 hours post-dose) on Days 1 and 92, Respectively for One of the Two Ipratropium-Controlled Studies*
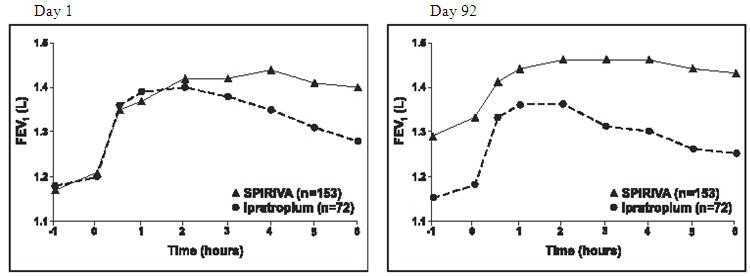
*Means adjusted for center, treatment, and baseline effect. On Day 92 (primary endpoint), a total of 151 and 69 patients in the SPIRIVA HandiHaler and ipratropium groups, respectively, completed through 3 months of observation. The data for the remaining patients were imputed using the last observation or least favorable observation carried forward.
A randomized, placebo-controlled clinical study in 105 patients with COPD demonstrated that bronchodilation was maintained throughout the 24-hour dosing interval in comparison to placebo, regardless of whether SPIRIVA HandiHaler was administered in the morning or in the evening.
Throughout each week of the one-year treatment period in the two placebo-controlled trials, patients taking SPIRIVA HandiHaler had a reduced requirement for the use of rescue short-acting beta2-agonists. Reduction in the use of rescue short-acting beta2-agonists, as compared to placebo, was demonstrated in one of the two 6-month studies.
4-Year Effects on
Lung Function
A 4-year, randomized, double-blind, placebo-controlled, multicenter clinical trial involving 5992
COPD patients was conducted to evaluate the long-term effects of SPIRIVA
HandiHaler on disease progression (rate of decline in FEV1).
Patients were permitted to use all respiratory medications (including
short-acting and long-acting beta-agonists, inhaled and systemic steroids, and
theophyllines) other than inhaled anticholinergics. The patients were 40 to 88
years of age, 75% male, and 90% Caucasian with a diagnosis of COPD and a mean pre-bronchodilator
FEV1 of 39% predicted (range = 9% to 76%) at study entry. There was
no difference between the groups in either of the co-primary efficacy
endpoints, yearly rate of decline in pre- and post-bronchodilator FEV1,
as demonstrated by similar slopes of FEV1 decline over time (Figure
3).
SPIRIVA HandiHaler maintained improvements in trough (pre-dose) FEV1 (adjusted means over time: 87 to 103 mL) throughout the 4 years of the study (Figure 3).
Figure 3 Trough (pre-dose) FEV1 Mean Values at Each Time Point
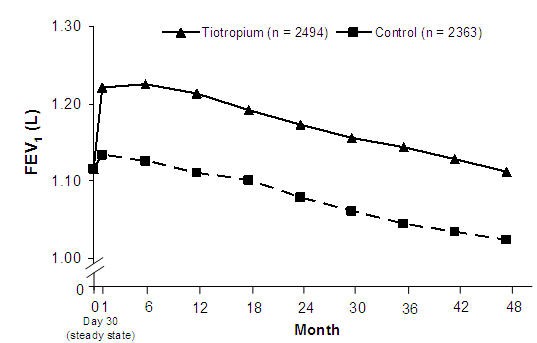
Repeated measure ANOVA was used to estimate means. Means are adjusted for baseline measurements. Baseline trough FEV1 (observed mean) = 1.12. Patients with ≥3 acceptable pulmonary function tests after Day 30 and non-missing baseline value were included in the analysis.
Exacerbations
The effect of SPIRIVA HandiHaler on COPD exacerbations was evaluated in two
clinical trials: a 4-year clinical trial described above and a 6-month clinical
trial of 1829 COPD patients in a Veterans Affairs setting. In the 6-month
trial, COPD exacerbations were defined as a complex of respiratory symptoms
(increase or new onset) of more than one of the following: cough, sputum,
wheezing, dyspnea, or chest tightness with a duration of at least 3 days
requiring treatment with antibiotics, systemic steroids, or hospitalization. The
population had an age ranging from 40 to 90 years with 99% males, 91%
Caucasian, and had COPD with a mean pre-bronchodilator FEV1 percent
predicted of 36% (range = 8% to 93%). Patients were permitted to use
respiratory medications (including short-acting and long-acting beta-agonists,
inhaled and systemic steroids, and theophyllines) other than inhaled
anticholinergics. In the 6-month trial, the co-primary endpoints were the
proportion of patients with COPD exacerbation and the proportion of patients
with hospitalization due to COPD exacerbation. SPIRIVA HandiHaler
significantly reduced the proportion of COPD patients who experienced
exacerbations compared to placebo (27.9% vs 32.3%, respectively; Odds Ratio (OR)
(tiotropium/placebo) = 0.81; 95% CI = 0.66, 0.99; p = 0.037). The proportion
of patients with hospitalization due to COPD exacerbations in patients who used
SPIRIVA HandiHaler compared to placebo was 7.0% vs 9.5%, respectively; OR =
0.72; 95% CI = 0.51, 1.01; p = 0.056.
Exacerbations were evaluated as a secondary outcome in the 4-year multicenter trial. In this trial, COPD exacerbations were defined as an increase or new onset of more than one of the following respiratory symptoms (cough, sputum, sputum purulence, wheezing, dyspnea) with a duration of three or more days requiring treatment with antibiotics and/or systemic (oral, intramuscular, or intravenous) steroids. SPIRIVA HandiHaler significantly reduced the risk of an exacerbation by 14% (Hazard Ratio (HR) = 0.86; 95% CI = 0.81, 0.91; p<0.001) and reduced the risk of exacerbation-related hospitalization by 14% (HR = 0.86; 95% CI = 0.78, 0.95; p<0.002) compared to placebo. The median time to first exacerbation was delayed from 12.5 months (95% CI = 11.5, 13.8) in the placebo group to 16.7 months (95% CI = 14.9, 17.9) in the SPIRIVA HandiHaler group.
16 HOW SUPPLIED/STORAGE AND HANDLING
SPIRIVA HandiHaler consists of SPIRIVA capsules and the HandiHaler device. SPIRIVA capsules contain 18 mcg of tiotropium and are light green, with the Boehringer Ingelheim company logo on the SPIRIVA capsule cap and TI 01 on the SPIRIVA capsule body, or vice versa.
The HandiHaler device is gray colored with a green piercing button. It is imprinted with SPIRIVA HandiHaler (tiotropium bromide inhalation powder), the Boehringer Ingelheim company logo, and the Pfizer company logo. It is also imprinted to indicate that SPIRIVA capsules should not be stored in the HandiHaler device and that the HandiHaler device is only to be used with SPIRIVA capsules.
SPIRIVA capsules are packaged in an aluminum/aluminum blister card and joined along a perforated-cut line. SPIRIVA capsules should always be stored in the blister and only removed immediately before use. The drug should be used immediately after the packaging over an individual SPIRIVA capsule is opened.
The following packages are available:
- carton containing 6 SPIRIVA capsules (5 unit-dose blister cards) and 1 HandiHaler inhalation device (NDC 54868-5109-0)
- carton containing 90 SPIRIVA capsules (9 unit-dose blister cards) and 1 HandiHaler inhalation device (NDC 54868-5109-1)
Storage
Store
at 25°C (77°F); excursions permitted to 15°–30°C (59°–86°F)
[see USP Controlled Room Temperature].
The SPIRIVA capsules should not be exposed to extreme temperature or moisture. Do not store SPIRIVA capsules in the HandiHaler device.
17 PATIENT COUNSELING INFORMATION
See FDA-approved Patient Labeling (17.6)
17.1 Instructions for Administering SPIRIVA HandiHaler
It is important for patients to understand how to correctly administer SPIRIVA capsules using the HandiHaler device [ see Patient Counseling Information (17.6) ]. Patients should be instructed that SPIRIVA capsules should only be administered via the HandiHaler device and the HandiHaler device should not be used for administering other medications. The contents of SPIRIVA capsules are for oral inhalation only and must not be swallowed .
SPIRIVA capsules should always be stored in sealed blisters. Only one SPIRIVA capsule should be removed immediately before use or its effectiveness may be reduced. Additional SPIRIVA capsules that are exposed to air (i.e., not intended for immediate use) should be discarded.
17.2 Paradoxical Bronchospasm
Patients should be informed that SPIRIVA HandiHaler can produce paradoxical bronchospasm. If paradoxical bronchospasm occurs, patients should discontinue SPIRIVA HandiHaler.
17.3 Urinary Retention
Difficulty passing urine and dysuria may be symptoms of new or worsening prostatic hyperplasia or bladder outlet obstruction. Patients should be instructed to consult a physician immediately should any of these signs or symptoms develop.
17.4 Visual Effects
Eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema may be signs of acute narrow-angle glaucoma. Patients should be told to consult a physician immediately should any of these signs and symptoms develop. Miotic eye drops alone are not considered to be effective treatment.
Patients should be told that care must be taken not to allow the powder to enter into the eyes as this may cause blurring of vision and pupil dilation.
17.5 Acute Exacerbation
Patients should understand that SPIRIVA HandiHaler is a once-daily maintenance bronchodilator and should not be used for immediate relief of breathing problems (i.e., as a rescue medication).
17.6 FDA-approved Patient Labeling
Patient Information and Patient’s Instructions for Use are supplied as tear-off leaflets following the full prescribing information and should be dispensed with each new prescription and refill.
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Marketed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
and
Pfizer Inc
New York, NY 10017 USA
Licensed from:
Boehringer Ingelheim International GmbH
Address medical inquiries to: (800) 542-6257 or (800) 459-9906 TTY.
SPIRIVA® and HandiHaler® are registered trademarks and are used under license from Boehringer Ingelheim International GmbH.
©Copyright 2009 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
SPIRIVA® (tiotropium bromide inhalation powder) is covered by U.S. Patent Nos. RE38,912, RE39,820, 5,478,578, 6,777,423, 6,908,928, 7,070,800, and 7,309,707 with other patents pending. The HandiHaler® inhalation device is covered by U.S. Design Patent No. D355,029 with other patents pending.
Rev: December 2009
IT1600WL1609
10004551/07
65626-08
Relabeling of "Additional Barcode" by:
Physicians Total Care, Inc.
Tulsa, OK 74146
Patient Information
SPIRIVA® (speh REE vah) HandiHaler®
(tiotropium bromide inhalation powder)
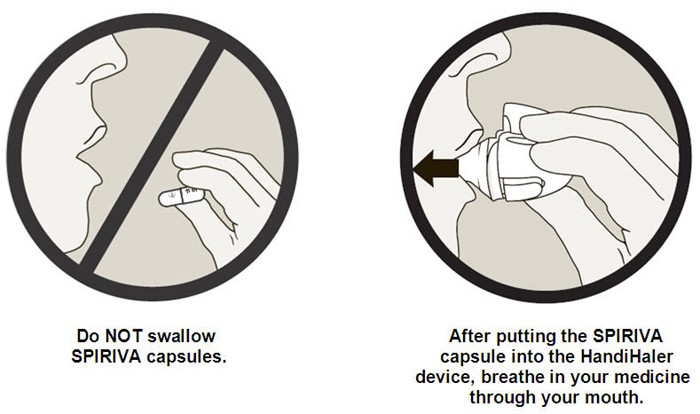
Important Information: Do not swallow SPIRIVA capsules. SPIRIVA capsules should only be used with the HandiHaler device. SPIRIVA HandiHaler should only be inhaled by mouth (oral inhalation).
Read the information that comes with your SPIRIVA HandiHaler before you start using it and each time you refill your prescription. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What is SPIRIVA HandiHaler?
SPIRIVA HandiHaler is a prescription medicine that you use one time every day (a maintenance medicine) to control symptoms of chronic obstructive pulmonary disease (COPD). SPIRIVA HandiHaler helps make your lungs work better for 24 hours. SPIRIVA HandiHaler relaxes your airways and helps keep them open. You may start to feel like it is easier to breathe on the first day, but it may take longer for you to feel the full effects of the medicine. SPIRIVA HandiHaler works best and may help make it easier to breathe when you use it every day.
SPIRIVA HandiHaler also reduces the likelihood of flare-ups and worsening of COPD symptoms (COPD exacerbations). A COPD exacerbation is defined as an increase or new onset of more than one COPD symptom such as cough, mucus, shortness of breath, and wheezing that requires medicine beyond your rescue medicine.
SPIRIVA HandiHaler is not a rescue medicine and should not be used for treating sudden breathing problems. Your doctor may give you other medicine to use for sudden breathing problems.
SPIRIVA HandiHaler has not been studied in children.
Who should not take SPIRIVA HandiHaler?
Do not use SPIRIVA HandiHaler if you:
- are allergic to tiotropium. See the end of this leaflet for a complete list of ingredients.
- have had an allergic reaction to ipratropium (Atrovent®).
Allergic reactions may include itching, rash, or swelling of the lips, tongue, or throat (trouble swallowing).
What should I tell my doctor before using SPIRIVA HandiHaler?
Before taking SPIRIVA HandiHaler, tell your doctor about all your medical conditions, including if you:
- have kidney problems.
- have glaucoma. SPIRIVA HandiHaler may make your glaucoma worse.
- have an enlarged prostate, problems passing urine, or a blockage in your bladder. SPIRIVA HandiHaler may make these problems worse.
- are pregnant or plan to become pregnant. It is not known if SPIRIVA HandiHaler could harm your unborn baby.
- are breast-feeding or plan to breast-feed. It is not known if SPIRIVA HandiHaler passes into breast milk. You and your doctor will decide if SPIRIVA HandiHaler is right for you while you breast-feed.
- have a severe allergy to milk proteins. Ask your doctor if you are not sure.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines and eye drops, vitamins, and herbal supplements. Some of your other medicines or supplements may affect the way SPIRIVA HandiHaler works. SPIRIVA HandiHaler is an anticholinergic medicine. You should not take other anticholinergic medicines while using SPIRIVA HandiHaler, including ipratropium. Ask your doctor or pharmacist if you are not sure if one of your medicines is an anticholinergic.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist when you get a new medicine.
How should I take SPIRIVA HandiHaler?
- Use SPIRIVA HandiHaler exactly as prescribed. Use SPIRIVA HandiHaler one time every day.
- Read the “Patient’s Instructions for Use” at the end of this leaflet before you use SPIRIVA HandiHaler. Talk with your doctor if you do not understand the instructions.
- Do not swallow SPIRIVA capsules.
- Only use SPIRIVA capsules with the HandiHaler device.
- Do not use the HandiHaler device to take any other medicine
- SPIRIVA HandiHaler comes as a powder in a SPIRIVA capsule that fits the HandiHaler device. Each SPIRIVA capsule, containing only a small amount of SPIRIVA powder, is one full dose of medicine.
- Separate one blister from the blister card. Then take out one of the SPIRIVA capsules from the blister package right before you use it.
- After the capsule is pierced, take a complete dose of SPIRIVA HandiHaler by breathing in the powder by mouth two times, using the HandiHaler device (take 2 inhalations from one SPIRIVA capsule). See the “Patient’s Instructions for Use” at the end of this leaflet.
- Throw away any SPIRIVA capsule that is not used right away after it is taken out of the blister package. Do not leave the SPIRIVA capsules open to air; they may not work as well.
- If you miss a dose, take it as soon as you remember. Do not use SPIRIVA HandiHaler more than one time every 24 hours.
- If you use more than your prescribed dose of SPIRIVA HandiHaler, call your doctor or a poison control center.
What should I avoid while using SPIRIVA HandiHaler?
Do not let the powder from the SPIRIVA capsule get into your eyes. Your vision may get blurry and the pupil in your eye may get larger (dilate). If this happens, call your doctor.
What are the possible side effects of SPIRIVA HandiHaler?
SPIRIVA HandiHaler can cause serious side effects. If you get any of the following side effects, stop taking SPIRIVA HandiHaler and get medical help right away.
- Allergic reaction. Symptoms may include: itching, rash, swelling of the lips, tongue, or throat (trouble swallowing).
- Sudden narrowing and blockage of the airways into the lungs (bronchospasm). Your breathing suddenly gets worse.
- New or worsened increased pressure in the eyes (acute narrow-angle glaucoma). Symptoms of acute narrow-angle glaucoma may include: eye pain, blurred vision, seeing halos (visual halos) or colored images along with red eyes.
- New or worsened urinary retention. Symptoms of blockage in your bladder and/or enlarged prostate may include: difficulty passing urine, painful urination.
Other side effects with SPIRIVA HandiHaler include:
- upper respiratory tract infection
- dry mouth
- sinus infection
- sore throat
- non-specific chest pain
- urinary tract infection
- indigestion
- runny nose
- constipation
- increased heart rate
- blurred vision
These are not all the possible side effects with SPIRIVA HandiHaler. Tell your doctor if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store SPIRIVA HandiHaler?
- Do not store SPIRIVA capsules in the HandiHaler device.
- Store SPIRIVA capsules in the sealed blister package at room temperature between 68°F–77°F (20°–25°C).
- Keep SPIRIVA capsules away from heat and cold (do not freeze).
- Store SPIRIVA capsules in a dry place. Throw away any unused SPIRIVA capsules that have been open to air.
Ask your doctor or pharmacist if you have any questions about storing your SPIRIVA capsules.
Keep SPIRIVA HandiHaler, SPIRIVA capsules, and all medicines out of the reach of children.
General information about SPIRIVA HandiHaler
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use SPIRIVA HandiHaler for a purpose for which it has not been prescribed. Do not give SPIRIVA HandiHaler to other people even if they have the same symptoms that you have. It may harm them.
For more information about SPIRIVA HandiHaler, talk with your doctor. You can ask your doctor or pharmacist for information about SPIRIVA HandiHaler that is written for health professionals.
For more information about SPIRIVA HandiHaler, you may call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257 or (TTY) 1-800-459-9906.
What are the ingredients in SPIRIVA HandiHaler?
Active ingredient: tiotropium
Inactive ingredient: lactose monohydrate
What is COPD (Chronic Obstructive Pulmonary Disease)?
COPD is a serious lung disease that includes chronic bronchitis, emphysema, or both. Most COPD is caused by smoking. When you have COPD, your airways become narrow. So, air moves out of your lungs more slowly. This makes it hard to breathe.
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Marketed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
and
Pfizer Inc
New York, NY 10017 USA
Licensed from:
Boehringer Ingelheim International GmbH
SPIRIVA® and HandiHaler® are registered trademarks and are used under license from Boehringer Ingelheim International GmbH.
©Copyright 2009 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
Rev: December 2009
IT1600WL1609
10004551/07
65626-08
Patient's Instructions for Use
SPIRIVA HandiHaler®
(tiotropium bromide inhalation powder)
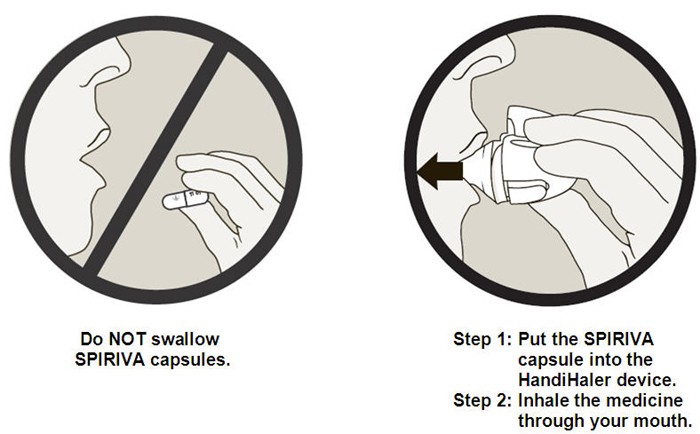
Important Information: Do not swallow SPIRIVA capsules. SPIRIVA capsules should only be used with the HandiHaler device. SPIRIVA HandiHaler should only be inhaled through your mouth (oral inhalation).
First read the Patient Information that comes with SPIRIVA HandiHaler for important information about using SPIRIVA HandiHaler.
Read these Patient's Instructions for Use before you start to use SPIRIVA HandiHaler and each time you refill your prescription. There may be new information.
For more information, ask your doctor or pharmacist.
SPIRIVA HandiHaler comes with SPIRIVA capsules and a HandiHaler device. The HandiHaler device is an inhalation device that is for use only with SPIRIVA capsules. Do not use the HandiHaler device to take any other medicine.
Becoming familiar with SPIRIVA HandiHaler:
|
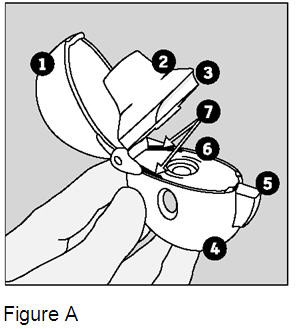
Remove the HandiHaler device from the pouch and become familiar with its components. (Figure A)
|
|
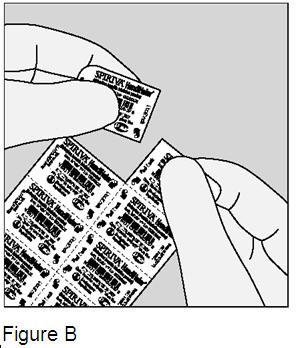
Each SPIRIVA capsule is packaged in a blister. Each blister can be separated from the blister card by tearing along the perforation. (Figure B) |
|
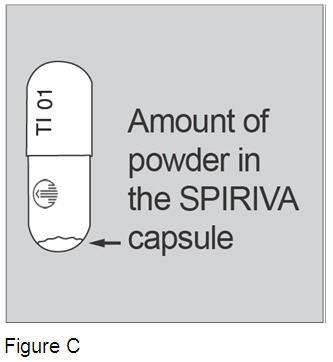
Do not open the SPIRIVA capsule before you insert it into the HandiHaler device. If you open the SPIRIVA capsule, it may not work. Each SPIRIVA capsule contains only a small amount of powder. (Figure C) This is one full dose. The product was designed this way. |
|
How do I inhale the contents of the SPIRIVA capsule using the HandiHaler device? Taking your dose of medicine using the HandiHaler device has four main steps:
(See below for details) |
|
|
Opening the HandiHaler device: |
|
|
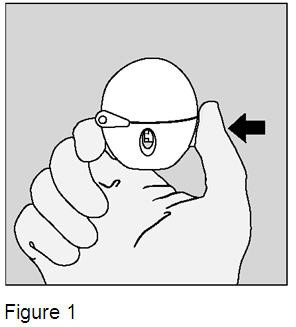
1. Open the dust cap by pressing the green piercing button. (Figure 1) |
|
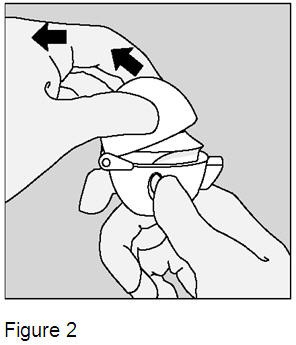
Pull the dust cap upwards to expose the mouthpiece. (Figure 2) |
|
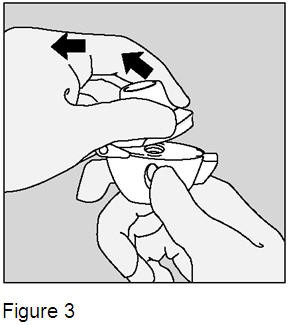
Open the mouthpiece by pulling the mouthpiece ridge upwards away from the base. (Figure 3) |
|
Removing a SPIRIVA capsule: |
|
|
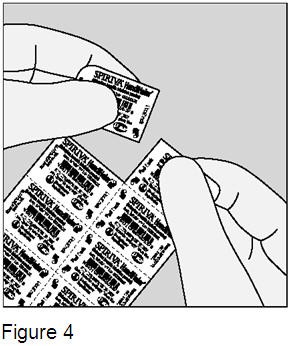
Before removing a SPIRIVA capsule from the blister, separate one of the blisters from the blister card by tearing along the perforation. (Figure 4)
Do not swallow SPIRIVA capsules. Always store SPIRIVA capsules in the sealed blisters. Remove only one SPIRIVA capsule from the blister right before use. Do not store SPIRIVA capsules in the HandiHaler device. Inhale the contents of the SPIRIVA capsule using the HandiHaler device right away after the blister packaging of an individual SPIRIVA capsule is opened, or else it may not work as well. |
|
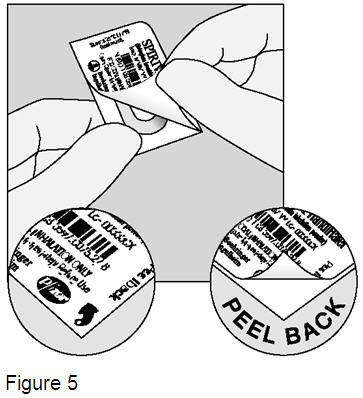
Right before you are ready to use your SPIRIVA HandiHaler: Bend back and forth one of the corners of the blister that has an arrow and then with your finger separate the aluminum foil layers. Carefully peel back the printed foil until you can see the whole SPIRIVA capsule. (Figure 5) Turn the blister upside down and tip the SPIRIVA capsule out, tapping the back of the blister, if needed.
Do not cut the foil or use sharp instruments to take out the SPIRIVA capsule from the blister. If more SPIRIVA capsules are opened to air, they should not be used and should be thrown away. |
|
|
|
|
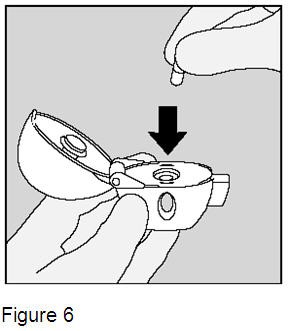
2. Insert (put) the SPIRIVA capsule in the center chamber of the HandiHaler device. It does not matter which end of the SPIRIVA capsule you put in the chamber. (Figure 6) |
|
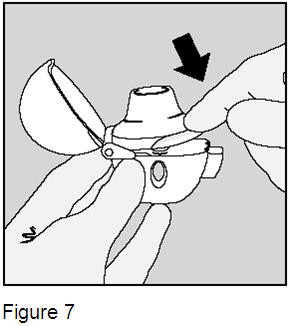
Close the mouthpiece until you hear a click, but leave the dust cap open. (Figure 7) |
|
|
|
 ` `
|
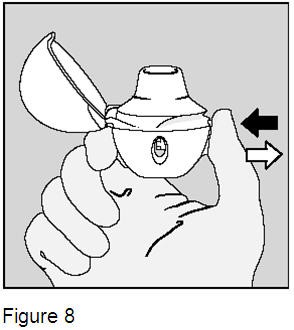
Hold the HandiHaler device with the mouthpiece upright. It is important that you hold the HandiHaler device in an upright position (Figure 8) when pressing the green piercing button.
3. Press the green piercing button until it is flat (flush) against the base, and release. This is how you make holes in the SPIRIVA capsule so that you get the medicine when you breathe in. Do not press the green button more than one time. |
|
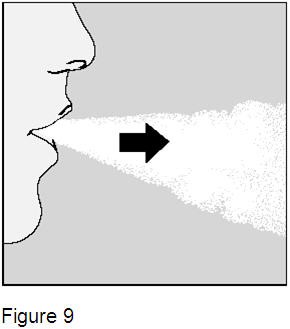
Breathe out completely. (Figure 9) Important: Do not breathe (exhale) into the mouthpiece of the HandiHaler device at any time. |
|
4. Breathe in (inhale)
|
|
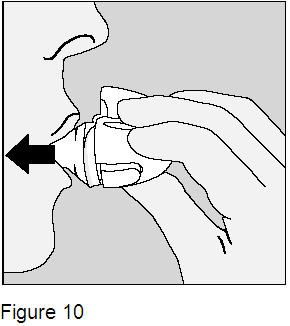
To make sure you get the full dose, you must breathe out completely, and inhale again as in step 4 above (Figure 10). Do not press the green piercing button again. |
|
|
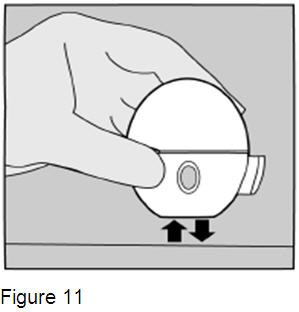
If you do not hear or feel the SPIRIVA capsule vibrate, do not press the green piercing button again. Instead, hold the HandiHaler device in an upright position and tap the HandiHaler device gently on a table. (Figure 11) Check to see that the mouthpiece is completely closed. Then, breathe in again – slowly and deeply. If you still do not hear or feel the SPIRIVA capsule vibrate after repeating the above steps, throw away the SPIRIVA capsule. Open the base by lifting the green piercing button and check the center chamber for pieces of the SPIRIVA capsule (SPIRIVA capsule fragments). SPIRIVA capsule fragments in the center chamber can cause a SPIRIVA capsule not to vibrate. Turn the HandiHaler device upside down and gently tap to remove the SPIRIVA capsule fragments. Call your doctor for instructions. |
|
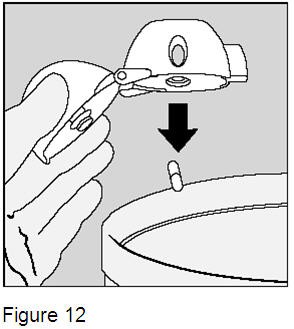
After you finish taking your daily dose of SPIRIVA HandiHaler, open the mouthpiece again. Tip out the used SPIRIVA capsule and throw it away. (Figure 12) |
|
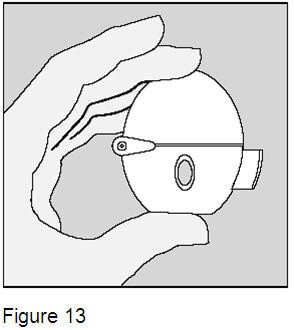
Close the mouthpiece and dust cap for storage of your HandiHaler device. (Figure 13) Do not store used or unused SPIRIVA capsules in the HandiHaler device. |
|
|
|
|
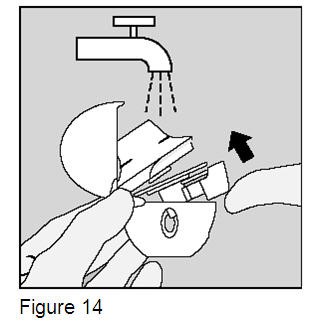
Clean the HandiHaler device one time each month or as needed. (Figure 14)
|
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Marketed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
and
Pfizer Inc
New York, NY 10017 USA
Licensed from:
Boehringer Ingelheim International GmbH
SPIRIVA® and HandiHaler® are registered trademarks and are used under license from Boehringer Ingelheim International GmbH
©Copyright 2009 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
Rev: December 2009
IT1600WL1609
10004551/07
65626-08
SPIRIVA HandiHaler
30 capsules (5 blister cards)
1 HandiHaler Inhalation Device

Spirivatiotropium bromide monohydrate CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||