Spironolactone
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- SPIRONOLACTONE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- SPIRONOLACTONE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- SPIRONOLACTONE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNINGSpironolactone has been shown to be a tumorigen in chronic toxicity studies in rats (seePRECAUTIONS). Spironolactone should be used only in those conditions described underINDICATIONS AND USAGE. Unnecessary use of this drug should be avoided.
SPIRONOLACTONE DESCRIPTION
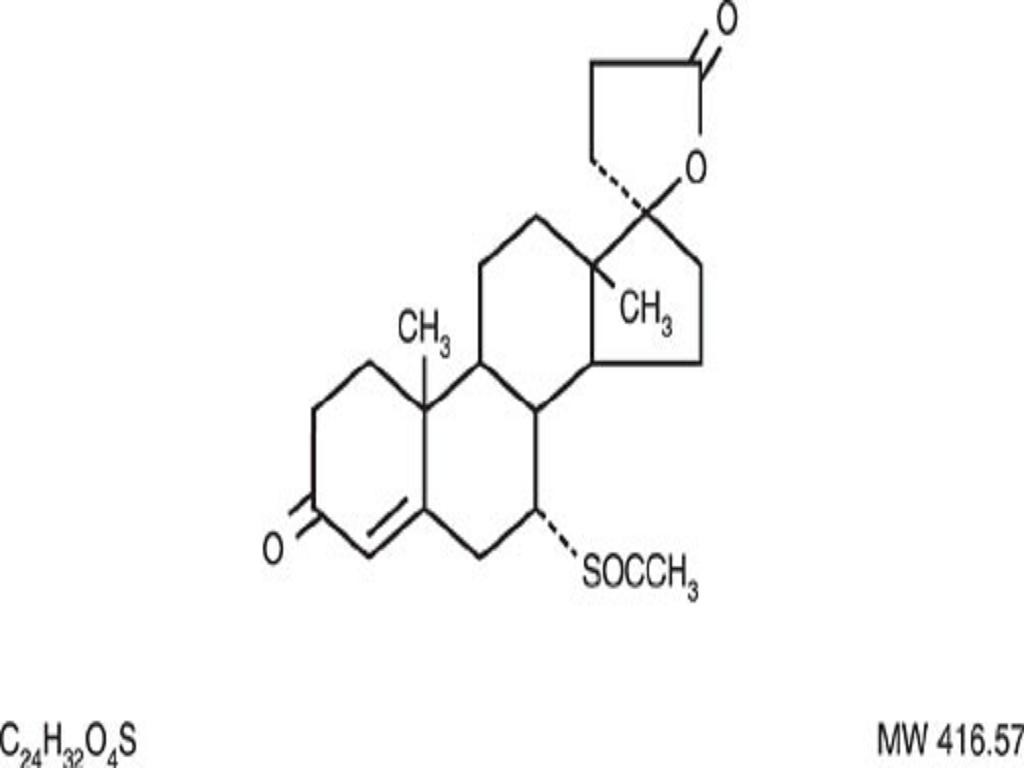
CLINICAL PHARMACOLOGY
Mechanism of Action.Aldosterone Antagonist Activity.
PHARMACOKINETICS
CLINICAL STUDIES
CLINICAL STUDIESSevere Heart Failure.
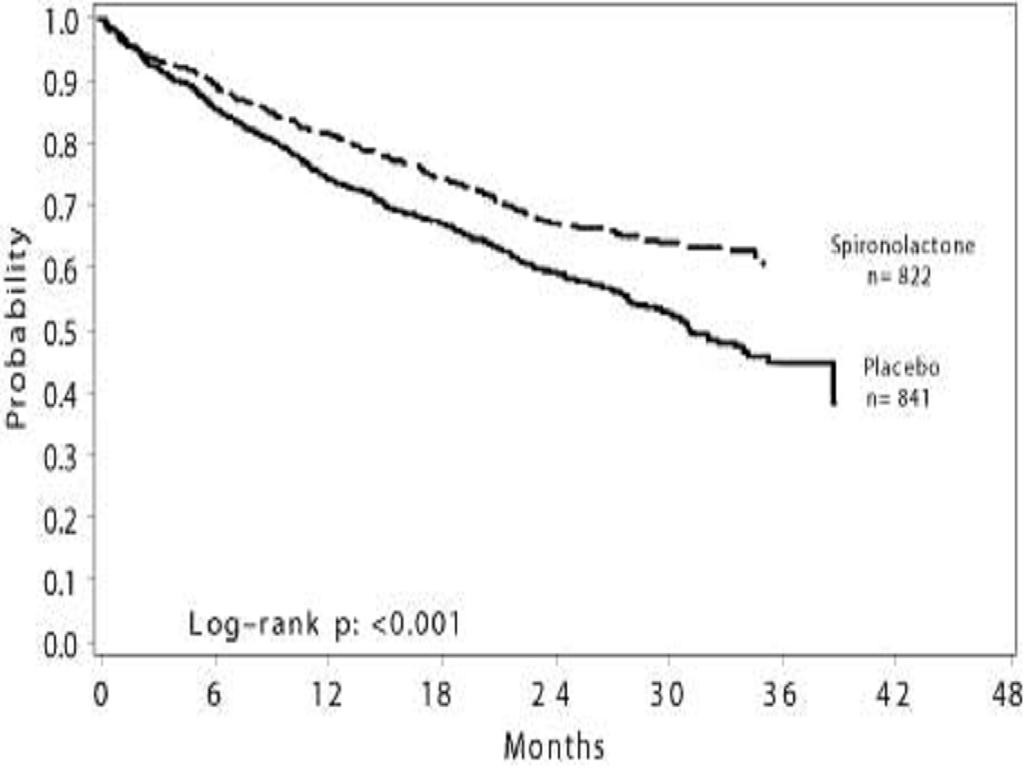
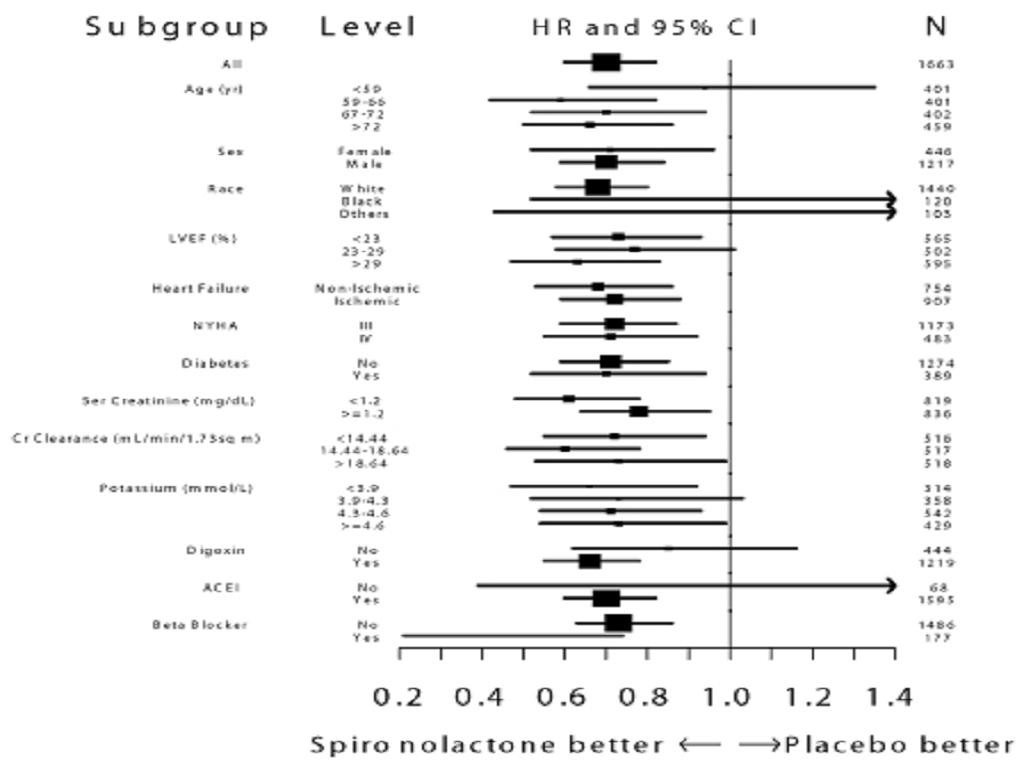
INDICATIONS & USAGE
Primary hyperaldosteronism for:
Edematous conditions for patients with:
Essential hypertension
Hypokalemia
Severe heart failure (NYHA class III - IV)
Usage in Pregnancy.
PRECAUTIONS: Pregnancy
There is hypervolemia during normal pregnancy which is not harmful to either the fetus or the mother (in the absence of cardiovascular disease), but which is associated with edema, including generalized edema, in the majority of pregnant women. If this edema produces discomfort, increased recumbency will often provide relief. In rare instances, this edema may cause extreme discomfort which is not relieved by rest. In these cases, a short course of diuretics may provide relief and may be appropriate.
SPIRONOLACTONE CONTRAINDICATIONS
WARNINGS
Potassium Supplementation.
PRECAUTIONS: General
Hyperkalemia in Patients with Severe Heart Failure.
CLINICAL STUDIES, Severe Heart FailureDOSAGE AND ADMINISTRATION, Severe Heart Failure
PRECAUTIONS: Drug Interactions
PRECAUTIONS
General.INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
ACE Inhibitors:Alcohol, Barbiturates, or Narcotics:
Corticosteroids, ACTH:
Pressor Amines (e.g., Norepinephrine):
Skeletal Muscle Relaxants, Nondepolarizing (e.g.,Tubocurarine):
Lithium:
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs):
Digoxin:
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects.Pregnancy Category C.
NURSING MOTHERS
PEDIATRIC USE
SPIRONOLACTONE ADVERSE REACTIONS
PRECAUTIONS
WARNINGSPRECAUTIONS
OVERDOSAGE
Treatment.
DOSAGE & ADMINISTRATION
Primary Hyperaldosteronism.Edema in Adults (Congestive Heart Failure, Hepatic Cirrhosis, or Nephrotic Syndrome).
Essential Hypertension.
Hypokalemia.
Severe Heart Failure (NYHA class IIIIV).
WARNINGS: Hyperkalemia in Patients with Severe Heart Failure
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

SpironolactoneSpironolactone TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!