Sulfamethoxazole and Trimethoprim
FULL PRESCRIBING INFORMATION: CONTENTS*
- SULFAMETHOXAZOLE AND TRIMETHOPRIM DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- SULFAMETHOXAZOLE AND TRIMETHOPRIM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- SULFAMETHOXAZOLE AND TRIMETHOPRIM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
SULFAMETHOXAZOLE AND TRIMETHOPRIM DESCRIPTION
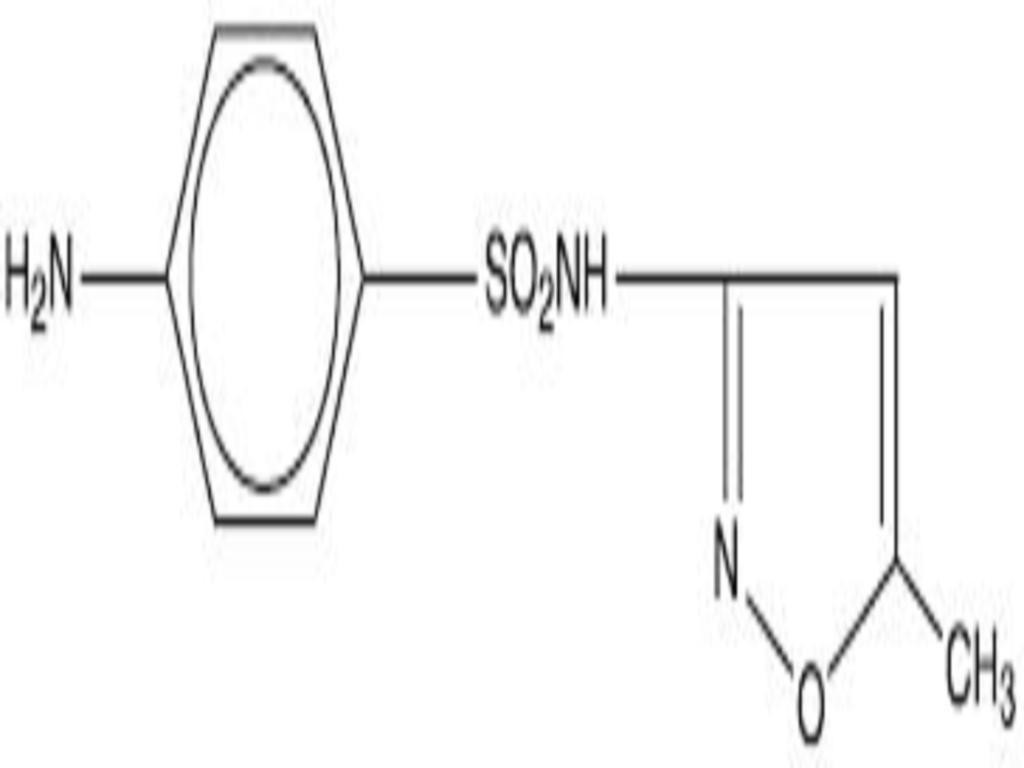
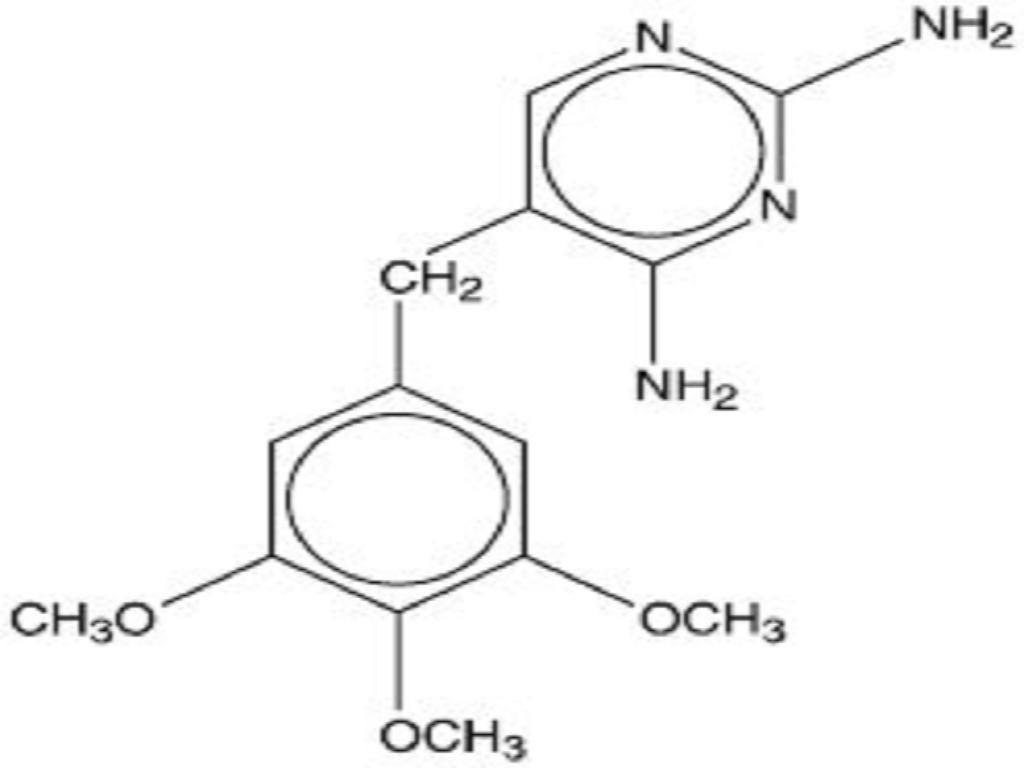
Inactive ingredients:
CLINICAL PHARMACOLOGY
DOSAGE AND ADMINISTRATION
Geriatric Pharmacokinetics:
Microbiology
INDICATIONS AND USAGE
Aerobic gram-positive microorganisms:
Aerobic gram-negative microorganisms:
Other Organisms:
Susceptibility Testing Methods:
Dilution Techniques:
**
Quality Control
**
Diffusion Techniques:
**
**
Quality Control
**
INDICATIONS & USAGE
Urinary Tract Infections:
Acute Otitis Media:
Acute Exacerbations of Chronic Bronchitis in Adults:
Shigellosis:
Pneumocystis Carinii Pneumonia:
Traveler's Diarrhea in Adults:
SULFAMETHOXAZOLE AND TRIMETHOPRIM CONTRAINDICATIONS
WARNINGS
FATALITIES ASSOCIATED WITH THE ADMINISTRATION OF SULFONAMIDES, ALTHOUGH RARE, HAVE OCCURRED DUE TO SEVERE REACTIONS, INCLUDING STEVENS-JOHNSON SYNDROME, TOXIC EPIDERMAL NECROLYSIS, FULMINANT HEPATIC NECROSIS, AGRANULOCYTOSIS, APLASTIC ANEMIA AND OTHER BLOOD DYSCRASIAS.SULFONAMIDES, INCLUDING SULFONAMIDE-CONTAINING PRODUCTS SUCH AS SULFAMETHOXAZOLE/TRIMETHOPRIM, SHOULD BE DISCONTINUED AT THE FIRST APPEARANCE OF SKIN RASH OR ANY SIGN OF ADVERSE REACTION.PRECAUTIONS
Cough, shortness of breath, and pulmonary infiltrates are hypersensitivity reactions of the respiratory tract that have been reported in association with sulfonamide treatment.
Thrombocytopenia
PRECAUTIONS
General:
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
Use in the Treatment of and Prophylaxis for Pneumocystis Carinii Pneumonia in Patients with Acquired Immunodeficiency Syndrome (AIDS):
WARNINGS
INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis:Mutagenesis:
Impairment of Fertility:
Teratogenic Effects: Pregnancy Category C.
Nonteratogenic Effects:
CONTRAINDICATIONS
NURSING MOTHERS
CONTRAINDICATIONSPEDIATRIC USE
INDICATIONS AND USAGECONTRAINDICATIONSGERIATRIC USE
WARNINGSADVERSE REACTIONSDOSAGE AND ADMINISTRATION
CLINICAL PHARMACOLOGY: Geriatric Pharmacokinetics
SULFAMETHOXAZOLE AND TRIMETHOPRIM ADVERSE REACTIONS
FATALITIES ASSOCIATED WITH THE ADMINISTRATION OF SULFONAMIDES, ALTHOUGH RARE, HAVE OCCURRED DUE TO SEVERE REACTIONS, INCLUDING STEVENS-JOHNSON SYNDROME, TOXIC EPIDERMAL NECROLYSIS, FULMINANT HEPATIC NECROSIS, AGRANULOCYTOSIS, APLASTIC ANEMIA AND OTHER BLOOD DYSCRASIAS (seeWARNINGSsection).PRECAUTIONS:Use in the Treatment of and Prophylaxis for Pneumocystis Carinii Pneumonia in Patients with Acquired Immunodeficiency Syndrome (AIDS)
WARNINGS
Postmarketing Experience
OVERDOSAGE
Acute:Chronic:
DOSAGE & ADMINISTRATION
Not recommended for use in pediatric patients less than 2 months of age.Urinary Tract Infections and Shigellosis in Adults and Pediatric Patients, and Acute Otitis Media in Children:
Adults:
Children:
For Patients with Impaired Renal Function:
Acute Exacerbations of Chronic Bronchitis in Adults:
Pneumocystis Carinii Pneumonia:
Treatment: Adults and Children:
Prophylaxis:
Adults:
Children:
Traveler's Diarrhea in Adults:
HOW SUPPLIED
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Sulfamethoxazole and TrimethoprimSulfamethoxazole and Trimethoprim TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!