Sulfamethoxazole and Trimethoprim
FULL PRESCRIBING INFORMATION: CONTENTS*
- Rx only
- SULFAMETHOXAZOLE AND TRIMETHOPRIM DESCRIPTION
- CLINICAL PHARMACOLOGY
- SULFAMETHOXAZOLE AND TRIMETHOPRIM INDICATIONS AND USAGE
- SULFAMETHOXAZOLE AND TRIMETHOPRIM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- Development of Drug Resistant Bacteria
- Use in the Treatment of and Prophylaxis for Pneumocystis Jiroveci Pneumonia in Patients with Acquired Immunodeficiency Syndrome (AIDS)
- Information for Patients
- Laboratory Tests
- Drug Interactions
- Drug/Laboratory Test Interactions
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Pregnancy
- Nursing Mothers
- Pediatric Use
- Geriatric Use
- SULFAMETHOXAZOLE AND TRIMETHOPRIM ADVERSE REACTIONS
- OVERDOSAGE
- SULFAMETHOXAZOLE AND TRIMETHOPRIM DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 400 mg/80 mg Bulk Tablet Label
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 800 mg/160 mg Bulk Tablet Label
FULL PRESCRIBING INFORMATION
Rx only
To reduce the development of drug-resistant bacteria and maintain the effectiveness of sulfamethoxazole and trimethoprim tablets and other antibacterial drugs, sulfamethoxazole and trimethoprim tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
SULFAMETHOXAZOLE AND TRIMETHOPRIM DESCRIPTION
N1101133

141843
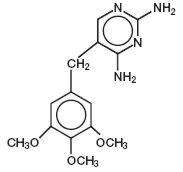
Inactive Ingredients:
CLINICAL PHARMACOLOGY
4
DOSAGE AND ADMINISTRATION 142
Geriatric Pharmacokinetics
3
Microbiology
In vitro
in vitro INDICATIONS AND USAGE
Aerobic gram-positive microorganisms
Streptococcus pneumoniae
Aerobic gram-negative microorganisms
Escherichia coli
Klebsiella
Enterobacter
Haemophilus influenzae
Morganella morganii
Proteus mirabilis
Proteus vulgaris
Shigella flexneri
Shigella sonnei
Other Organisms
Pneumocystis jiroveci
Susceptibility Testing Methods
Dilution Techniques
4
Enterobacteriaceae:
Haemophilus influenzaea Streptococcus pneumoniaeb
b
a.Haemophilus influenzae Haemophilus 4
b. 4
Quality Control
Escherichia coli
Haemophilus influenzaec
Streptococcus pneumoniaed
c.Haemophilus influenzaeHaemophilus4
d.4
Diffusion Techniques
5
Enterobacteriaceae Haemophilus influenzaee
e.Haemophilus influenzae Haemophilus 5
Streptococcus pneumoniaef
f.2.5
Quality Control
Escherichia coli
Haemophilus influenzaeg
Streptococcus pneumoniaeh
Enterococcus faecalis
g.Haemophilus influenzae Haemophilus 5
h.2.5
SULFAMETHOXAZOLE AND TRIMETHOPRIM INDICATIONS AND USAGE
Urinary Tract Infections
Escherichia coli, Klebsiella Enterobacter Morganella morganii, Proteus mirabilis Proteus vulgaris.
Acute Otitis Media
Streptococcus pneumoniae Haemophilus influenzae
Acute Exacerbations of Chronic Bronchitis in Adults
Streptococcus pneumoniae Haemophilus influenzae
Shigellosis
Shigella flexneri Shigella sonnei
Pneumocystis Jiroveci Pneumonia
Pneumocystis jiroveci Pneumocystis jiroveci Pneumocystis jiroveci
Traveler's Diarrhea in Adults
E. coli.
SULFAMETHOXAZOLE AND TRIMETHOPRIM CONTRAINDICATIONS
WARNINGS
Hypersensitivity and Other Fatal Reactions
FATALITIES ASSOCIATED WITH THE ADMINISTRATION OF SULFONAMIDES, ALTHOUGH RARE, HAVE OCCURRED DUE TO SEVERE REACTIONS, INCLUDING STEVENS-JOHNSON SYNDROME, TOXIC EPIDERMAL NECROLYSIS, FULMINANT HEPATIC NECROSIS, AGRANULOCYTOSIS, APLASTIC ANEMIA AND OTHER BLOOD DYSCRASIAS.
SULFONAMIDES, INCLUDING SULFONAMIDE-CONTAINING PRODUCTS SUCH AS SULFAMETHOXAZOLE AND TRIMETHOPRIM, SHOULD BE DISCONTINUED AT THE FIRST APPEARANCE OF SKIN RASH OR ANY SIGN OF ADVERSE REACTION. PRECAUTIONS
Cough, shortness of breath, and pulmonary infiltrates are hypersensitivity reactions of the respiratory tract that have been reported in association with sulfonamide treatment.
Thrombocytopenia
Streptococcal Infections and Rheumatic Fever
Clostridium Difficile Associated Diarrhea
Clostridium difficile C. difficile
C. difficile C. difficile
C. difficile C. difficile,
Adjunctive Treatment with Leucovorin for Pneumocystis jiroveci Pneumonia
Pneumocystis jiroveci 6 Pneumocystis jiroveci
PRECAUTIONS
Development of Drug Resistant Bacteria
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
Use in the Treatment of and Prophylaxis for Pneumocystis Jiroveci Pneumonia in Patients with Acquired Immunodeficiency Syndrome (AIDS)
Pneumocystis jiroveci 7 WARNINGS).
Pneumocystis jiroveci WARNINGS
Pneumocystis jiroveci
Information for Patients
Laboratory Tests
Drug Interactions
8,9
Drug/Laboratory Test Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Mutagenesis
in vitro
Impairment of Fertility
Pregnancy
Teratogenic Effects
Pregnancy Category C
10
Nonteratogenic Effects
CONTRAINDICATIONS
Nursing Mothers
CONTRAINDICATIONS
Pediatric Use
INDICATIONS CONTRAINDICATIONS
Geriatric Use
WARNINGS ADVERSE REACTIONS DOSAGE AND ADMINISTRATION
CLINICAL PHARMACOLOGY: Geriatric Pharmacokinetics
SULFAMETHOXAZOLE AND TRIMETHOPRIM ADVERSE REACTIONS
FATALITIES ASSOCIATED WITH THE ADMINISTRATION OF SULFONAMIDES, ALTHOUGH RARE, HAVE OCCURRED DUE TO SEVERE REACTIONS, INCLUDING STEVENS-JOHNSON SYNDROME, TOXIC EPIDERMAL NECROLYSIS, FULMINANT HEPATIC NECROSIS, AGRANULOCYTOSIS, APLASTIC ANEMIA AND OTHER BLOOD DYSCRASIAS (SEE WARNINGS SECTION).
Hematologic
Allergic Reactions
Gastrointestinal
Genitourinary
Metabolic and Nutritional
PRECAUTIONS: Use in the Treatment of and Prophylaxis for Pneumocystis Jiroveci Pneumonia in Patients with Acquired Immunodeficiency Syndrome (AIDS)
Neurologic
Psychiatric
Endocrine
Musculoskeletal
Respiratory
WARNINGS
Miscellaneous
Postmarketing Experience
- Thrombotic thrombocytopenia purpura
- Idiopathic thrombocytopenic purpura
OVERDOSAGE
Acute
Chronic
SULFAMETHOXAZOLE AND TRIMETHOPRIM DOSAGE AND ADMINISTRATION
Urinary Tract Infections and Shigellosis in Adults and Pediatric Patients, and Acute Otitis Media in Children
Adults
Children
Children 2 months of age or older:
| Weight |
Dose–every 12 hours |
|
| lb |
kg |
Tablets |
| 22 44 66 88 |
10 20 30 40 |
– 1 1½ 2 or 1 DS tablet |
For Patients with Impaired Renal Function
| Creatinine Clearance (mL/min) |
Recommended Dosage Regimen |
| Above 30 15–30 Below 15 |
Usual standard regimen ½ the usual regimen Use not recommended |
Acute Exacerbations of Chronic Bronchitis in Adults
Pneumocystis Jiroveci Pneumonia
Treatment
Adults and Children
Pneumocystis jiroveci 11
| Weight |
Dose–every 6 hours |
|
| lb |
kg |
Tablets |
| 18 35 53 70 88 106 141 176 |
8 16 24 32 40 48 64 80 |
– 1 1½ 2 or 1 DS tablet 2½ 3 or 1½ DS tablets 4 or 2 DS tablets 5 or 2½ DS tablets |
Prophylaxis
Adults
12
Children
2213
| Body Surface Area |
Dose–every 12 hours |
| (m2) |
Tablets |
| 0.26 0.53 1.06 |
– ½ 1 |
Traveler's Diarrhea in Adults
HOW SUPPLIED
Sulfamethoxazole and Trimethoprim Tablets USP, 400 mg/80 mg
Sulfamethoxazole and Trimethoprim Tablets USP, 800 mg/160 mg
Store at
REFERENCES
- Kremers P, Duvivier J, Heusghem C. Pharmacokinetic Studies of Co-Trimoxazole in Man after Single and Repeated Doses. J Clin Pharmacol. Feb-Mar 1974; 14:112–117.
- Kaplan SA, et al. Pharmacokinetic Profile of Trimethoprim-Sulfamethoxazole in Man. J Infect Dis. Nov 1973; 128 (Suppl): S547–S555.
- Varoquaux O, et al. Pharmacokinetics of the trimethoprim-sulfamethoxazole combination in the elderly. Br J Clin Pharmacol. 1985;20:575–581.
- Rudoy RC, Nelson JD, Haltalin KC. Antimicrobial Agents Chemother. May 1974;5:439–443.
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard – Fourth Edition. NCCLS Document M7–A4, Vol.17, No. 2, NCCLS, Wayne, PA, January, 1997.
- Safrin S, Lee BL, Sande MA. Adjunctive folinic acid with trimethoprim-sulfamethoxazole for Pneumocystis carinii pneumonia in AIDS patients is associated with an increased risk of therapeutic failure and death. J Infect Dis. 1994 Oct;170(4):912-7.
- Hardy DW, et al. A controlled trial of trimethoprim-sulfamethoxazole or aerosolized pentamidine for secondary prophylaxis of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992; 327: 1842–1848.
- Marinella Mark A. 1999. Trimethoprim-induced hyperkalemia: An analysis of reported cases. Gerontol. 45:209–212.
- Margassery, S. and B. Bastani. 2002. Life threatening hyperkalemia and acidosis secondary to trimethoprim-sulfamethoxazole treatment. J. Nephrol. 14:410–414.
- Brumfitt W, Pursell R. Trimethoprim/Sulfamethoxazole in the Treatment of Bacteriuria in Women. J Infect Dis. Nov 1973; 128 (Suppl):S657–S663.
- Masur H. Prevention and treatment of Pneumocystis pneumonia. N Engl J Med. 1992; 327: 1853–1880.
- Recommendations for prophylaxis against Pneumocystis carinii pneumonia for adults and adolescents infected with human immunodeficiency virus. MMWR. 1992; 41(RR-4):1–11.
- CDC Guidelines for prophylaxis against Pneumocystis carinii pneumonia for children infected with human immunodeficiency virus. MMWR. 1991; 40(RR-2):1–13.
Manufactured for:
Aurobindo Pharma USA, Inc.
2400 Route 130 North
Dayton, NJ 08810
Manufactured by:
Aurobindo Pharma Limited
Unit-VII (SEZ)
Mahaboob Nagar (Dt)
AP-509302, INDIA
Revised: 09/2012
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 400 mg/80 mg Bulk Tablet Label
3000 Tablets
Batch :
Mfg :
Expiry :
To be repacked within six months from the date of manufacturing
NDC 65862-419-39
BULK SHIPMENT
PLEASE HANDLE CAREFULLY
Rx only
Sulfamethoxazole and Trimethoprim Tablets, USP
400 mg/80 mg
Each tablet contains:
Sulfamethoxazole USP .........................................400 mg
Trimethoprim USP ....................................................80 mg
CAUTION: FOR REPACKAGING ONLY
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15°
to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Manufactured by:
Aurobindo Pharma Limited
Unit-VII (SEZ)
Mahaboob Nagar (Dt)
AP-509302, INDIA
M.L.No.: 22/MN/AP/2009/F/G

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 800 mg/160 mg Bulk Tablet Label
1500 Tablets
BULK SHIPMENT
PLEASE HANDLE CAREFULLY
Rx only
Sulfamethoxazole and Trimethoprim Tablets, USP
800 mg/160 mg
DOUBLE STRENGTH
Each tablet contains:
CAUTION:
Store at
Aurobindo Pharma Limited

Sulfamethoxazole and TrimethoprimSulfamethoxazole and Trimethoprim TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Sulfamethoxazole and TrimethoprimSulfamethoxazole and Trimethoprim TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||