Sulfamethoxazole and Trimethoprim
Sulfamethoxazole and Trimethoprim Tablets and Double Strength Tablets, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- SULFAMETHOXAZOLE AND TRIMETHOPRIM DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- SULFAMETHOXAZOLE AND TRIMETHOPRIM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- SULFAMETHOXAZOLE AND TRIMETHOPRIM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- SPL UNCLASSIFIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
SULFAMETHOXAZOLE AND TRIMETHOPRIM DESCRIPTION
Sulfamethoxazole and trimethoprim is a synthetic antibacterial combination product available in DS (double strength) tablets, each containing 800 mg sulfamethoxazole, USP and 160 mg trimethoprim, USP; in tablets, each containing 400 mg sulfamethoxazole, USP and 80 mg trimethoprim, USP for oral administration.
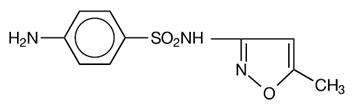
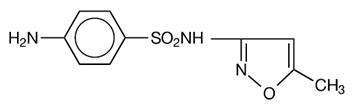
Sulfamethoxazole, USP is N 1-(5-methyl-3-isoxazolyl) sulfanilamide; the molecular formula is C10H11N3O3S. It is almost white, odorless, tasteless compound with a molecular weight of 253.28 and the following structural formula:
Trimethoprim, USP is 2,4-diamino-5-(3,4,5-trimethoxybenzyl) pyrimidine; the molecular formula is C14H18N4O3. It is a white to light yellow, odorless, bitter compound with a molecular weight of 290.3. It has the following structural formula:
Inactive Ingredients: Magnesium stearate, povidone, pregelatinized starch and sodium starch glycolate.
CLINICAL PHARMACOLOGY
Sulfamethoxazole and trimethoprim is rapidly absorbed following oral administration. Both sulfamethoxazole and trimethoprim exist in the blood as unbound, protein-bound and metabolized forms; sulfamethoxazole also exists as the conjugated form. The metabolism of sulfamethoxazole occurs predominately by N4-acetylation, although the glucuronide conjugate has been identified. The principal metabolites of trimethoprim are the 1- and 3-oxides and the 3'- and 4'-hydroxy derivatives. The free forms of sulfamethoxazole and trimethoprim are considered to be the therapeutically active forms. Approximately 70% of sulfamethoxazole and 44% of trimethoprim are bound to plasma proteins. The presence of 10 mg percent of sulfamethoxazole in plasma decreases the protein binding of trimethoprim by an insignificant degree; trimethoprim does not influence the protein binding of sulfamethoxazole.
Peak blood levels for the individual components occur 1 to 4 hours after oral administration. The mean serum half-lives of sulfamethoxazole and trimethoprim are 10 and 8 to 10 hours, respectively. However, patients with severely impaired renal function exhibit an increase in the half-lives of both components, requiring dosage regimen adjustment (see DOSAGE AND ADMINISTRATION section). Detectable amounts of sulfamethoxazole and trimethoprim are present in the blood 24 hours after drug administration. During administration of 800 mg sulfamethoxazole and 160 mg trimethoprim b.i.d., the mean steady-state plasma concentration of trimethoprim was 1.72 mcg/mL. The steady-state mean plasma levels of free and total sulfamethoxazole were 57.4 mcg/mL and 68 mcg/mL, respectively. These steady-state levels were achieved after three days of drug administration.1 Excretion of sulfamethoxazole and trimethoprim is primarily by the kidneys through both glomerular filtration and tubular secretion. Urine concentrations of both sulfamethoxazole and trimethoprim are considerably higher than are the concentrations in the blood. The average percentage of the dose recovered in urine from 0 to 72 hours after a single oral dose of sulfamethoxazole and trimethoprim is 84.5% for total sulfonamide and 66.8% for free trimethoprim. Thirty percent of the total sulfonamide is excreted as free sulfamethoxazole, with the remaining as N4-acetylated metabolite.2 When administered together as sulfamethoxazole and trimethoprim, neither sulfamethoxazole nor trimethoprim affects the urinary excretion pattern of the other.
Both sulfamethoxazole and trimethoprim distribute to sputum, vaginal fluid and middle ear fluid; trimethoprim also distributes to bronchial secretion, and both pass the placental barrier and are excreted in human milk.
Geriatric Pharmacokinetics: The pharmacokinetics of sulfamethoxazole 800 mg and trimethoprim 160 mg were studied in 6 geriatric subjects (mean age: 78.6 years) and 6 young healthy subjects (mean age: 29.3 years) using a non-US approved formulation. Pharmacokinetic values for sulfamethoxazole in geriatric subjects were similar to those observed in young adult subjects. The mean renal clearance of trimethoprim was significantly lower in geriatric subjects compared with young adult subjects (19 mL/h/kg vs. 55 mL/h/kg). However, after normalizing by body weight, the apparent total body clearance of trimethoprim was on average 19% lower in geriatric subjects compared with young adult subjects.3
Microbiology
Sulfamethoxazole inhibits bacterial synthesis of dihydrofolic acid by competing with para-aminobenzoic acid (PABA). Trimethoprim blocks the production of tetrahydrofolic acid from dihydrofolic acid by binding to and reversibly inhibiting the required enzyme, dihydrofolate reductase. Thus, sulfamethoxazole and trimethoprim blocks two consecutive steps in the biosynthesis of nucleic acids and proteins essential to many bacteria.
In vitro studies have shown that bacterial resistance develops more slowly with both sulfamethoxazole and trimethoprim in combination than with either sulfamethoxazole or trimethoprim alone.
Sulfamethoxazole and trimethoprim have been shown to be active against most strains of the following microorganisms, both In vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobic Gram-Positive Microorganisms:
Streptococcus pneumoniae
Aerobic Gram-Negative Microorganisms:
Escherichia coli (including susceptible enterotoxigenic strains implicated in traveler’s diarrhea)
Klebsiella species
Enterobacter species
Haemophilus influenzae
Morganella morganii
Proteus mirabilis
Proteus vulgaris
Shigella flexneri
Shigella sonnei
Other Organisms:
Pneumocystis jiroveci
Susceptibility Testing Methods
Dilution Techniques:
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method4 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of sulfamethoxazole/trimethoprim powder. The MIC values should be interpreted according to the following criteria:
| For testing Enterobacteriaceae: | |
| MIC (mcg/mL) | Interpretation |
| ≤2/38 | Susceptible (S) |
| ≥4/76 | Resistant (R) |
| When testing either Haemophilus influenzae a or Streptococcus pneumoniae b | |
| MIC (mcg/mL) | Interpretationb |
| ≤0.5/9.5 | Susceptible (S) |
| 1/19 – 2/38 | Intermediate |
| ≥4/76 | Resistant (R) |
A report of “Susceptible” indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of “Intermediate” indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Quality Control
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard sulfamethoxazole/trimethoprim powder should provide the following range of values:
| Microorganism | MIC (mcg/mL) | |
| Escherichia coli | ATCC 25922 | ≤0.5/9.5 |
| Haemophilus influenzae c | ATCC 49247 | 0.03/0.59 – 0.25/4.75 |
| Streptococcus pneumoniae d | ATCC 49619 | 0.12/2.4 – 1/19 |
Diffusion Techniques:
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure5 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 1.25/23.75 mcg of sulfamethoxazole/trimethoprim to test the susceptibility of microorganisms to sulfamethoxazole/trimethoprim.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 1.25/23.75 mcg of sulfamethoxazole/trimethoprim disk should be interpreted according to the following criteria:
| For testing either Enterobacteriaceae or Haemophilus influenzae e: | |
| Zone Diameter (mm) | Interpretation |
| ≥16 | Susceptible (S) |
| 11 to 15 | Intermediate (I) |
| ≤10 | Resistant (R) |
| e. These zone diameter standards are applicable only for disk diffusion testing with Haemophilus influenzae and Haemophilus Test Medium (HTM).5 | |
| When testing Streptococcus pneumoniae f | |
| Zone Diameter (mm) | Interpretation |
| ≥19 | Susceptible (S) |
| 16 to 18 | Intermediate (I) |
| ≤15 | Resistant (R) |
| f. These zone diameter interpretive standards are applicable only to tests performed using Mueller-Hinton agar supplemented with 5% defibrinated sheep blood when incubated at 5% CO2.5 |
Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for sulfamethoxazole/trimethoprim.
Quality Control
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 1.25/23.75 mcg sulfamethoxazole/trimethoprim disk* should provide the following zone diameters in these laboratory test quality control strains:
| Microorganism | Zone Diameter Ranges (mm) | |
| Escherichia coli | ATCC 25922 | 23 to 29 |
| Haemophilus influenzae g | ATCC 49247 | 24 to 32 |
| Streptococcus pneumoniae h | ATCC 49619 | 20 to 28 |
INDICATIONS & USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of sulfamethoxazole and trimethoprim tablets, USP and other antibacterial drugs, sulfamethoxazole and trimethoprim tablets, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Urinary Tract Infections: For the treatment of urinary tract infections due to susceptible strains of the following organisms: Escherichia coli, Klebsiella species, Enterobacter species, Morganella morganii, Proteus mirabilis and Proteus vulgaris. It is recommended that initial episodes of uncomplicated urinary tract infections be treated with a single effective antibacterial agent rather than the combination.
Acute Otitis Media: For the treatment of acute otitis media in pediatric patients due to susceptible strains of Streptococcus pneumoniae or Haemophilus influenzae when in the judgment of the physician sulfamethoxazole and trimethoprim offers some advantage over the use of other antimicrobial agents. To date, there are limited data on the safety of repeated use of sulfamethoxazole and trimethoprim in pediatric patients under two years of age. Sulfamethoxazole and trimethoprim is not indicated for prophylactic or prolonged administration in otitis media at any age.
Acute Exacerbations of Chronic Bronchitis in Adults: For the treatment of acute exacerbations of chronic bronchitis due to susceptible strains of Streptococcus pneumoniae or Haemophilus influenzae when in the judgment of the physician sulfamethoxazole and trimethoprim offers some advantage over the use of a single antimicrobial agent.
Shigellosis: For the treatment of enteritis caused by susceptible strains of Shigella flexneri and Shigella sonnei when antibacterial therapy is indicated.
Pneumocystis Jiroveci Pneumonia: For the treatment of documented Pneumocystis jiroveci pneumonia and for prophylaxis against Pneumocystis jiroveci pneumonia in individuals who are immunosuppressed and considered to be at an increased risk of developing Pneumocystis jiroveci pneumonia.
Traveler’s Diarrhea in Adults: For the treatment of traveler’s diarrhea due to susceptible strains of enterotoxigenic E. coli.
SULFAMETHOXAZOLE AND TRIMETHOPRIM CONTRAINDICATIONS
Sulfamethoxazole and trimethoprim is contraindicated in patients with a known hypersensitivity to trimethoprim, USP or sulfonamides, in patients with a history of drug-induced immune thrombocytopenia with use of trimethoprim, USP and/or sulfonamides, and in patients with documented megaloblastic anemia due to folate deficiency. Sulfamethoxazole and trimethoprim is also contraindicated in pregnant patients and nursing mothers, because sulfonamides pass the placenta and are excreted in the milk and may cause kernicterus. Sulfamethoxazole and trimethoprim is contraindicated in pediatric patients less than 2 months of age. Sulfamethoxazole and trimethoprim is also contraindicated in patients with marked hepatic damage or with severe renal insufficiency when renal function status cannot be monitored.
WARNINGS
Hypersensitivity and Other Fatal Reactions
FATALITIES ASSOCIATED WITH THE ADMINISTRATION OF SULFONAMIDES, ALTHOUGH RARE, HAVE OCCURRED DUE TO SEVERE REACTIONS, INCLUDING STEVENS-JOHNSON SYNDROME, TOXIC EPIDERMAL NECROLYSIS, FULMINANT HEPATIC NECROSIS, AGRANULOCYTOSIS, APLASTIC ANEMIA AND OTHER BLOOD DYSCRASIAS.
SULFONAMIDES, INCLUDING SULFONAMIDE-CONTAINING PRODUCTS SUCH AS SULFAMETHOXAZOLE/TRIMETHOPRIM, SHOULD BE DISCONTINUED AT THE FIRST APPEARANCE OF SKIN RASH OR ANY SIGN OF ADVERSE REACTION. In rare instances, a skin rash may be followed by a more severe reaction, such as Stevens-Johnson syndrome, toxic epidermal necrolysis, hepatic necrosis and serious blood disorders (see PRECAUTIONS ). Clinical signs, such as rash, sore throat, fever, arthralgia, pallor, purpura or jaundice may be early indications of serious reactions.
Cough, shortness of breath and pulmonary infiltrates are hypersensitivity reactions of the respiratory tract that have been reported in association with sulfonamide treatment.
Thrombocytopenia
Sulfamethoxazole/trimethoprim-induced thrombocytopenia may be an immune-mediated disorder. Severe cases of thrombocytopenia that are fatal or life threatening have been reported. Thrombocytopenia usually resolves within a week upon discontinuation of sulfamethoxazole and trimethoprim.
Streptococcal Infections and Rheumatic Fever
The sulfonamides should not be used for treatment of group A β-hemolytic streptococcal infections. In an established infection, they will not eradicate the streptococcus and, therefore, will not prevent sequelae such as rheumatic fever.
Clostridium difficile associated diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including sulfamethoxazole and trimethoprim, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile and surgical evaluation should be instituted as clinically indicated.
Adjunctive treatment with Leucovorin for Pneumocystis jiroveci pneumonia
Treatment failure and excess mortality were observed when trimethoprim-sulfamethoxazole was used concomitantly with leucovorin for the treatment of HIV positive patients with Pneumocystis jiroveci pneumonia in a randomized placebo controlled trial.6 Co-administration of trimethoprim-sulfamethoxazole and leucovorin during treatment of Pneumocystis jiroveci pneumonia should be avoided.
PRECAUTIONS
Development of drug resistant bacteria
Prescribing sulfamethoxazole and trimethoprim tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Folate deficiency
Sulfamethoxazole and trimethoprim should be given with caution to patients with impaired renal or hepatic function, to those with possible folate deficiency (e.g., the elderly, chronic alcoholics, patients receiving anticonvulsant therapy, patients with malabsorption syndrome and patients in malnutrition states) and to those with severe allergies or bronchial asthma.
Hematological changes indicative of folic acid deficiency may occur in elderly patients or in patients with preexisting folic acid deficiency or kidney failure. These effects are reversible by folinic acid therapy.
Hemolysis
In glucose-6-phosphate dehydrogenase deficient individuals, hemolysis may occur. This reaction is frequently dose-related. (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION ).
Hypoglycemia
Cases of hypoglycemia in non-diabetic patients treated with sulfamethoxazole and trimethoprim are seen rarely, usually occurring after a few days of therapy. Patients with renal dysfunction, liver disease, malnutrition or those receiving high doses of sulfamethoxazole and trimethoprim are particularly at risk.
Phenylalanine metabolism
Trimethoprim has been noted to impair phenylalanine metabolism but this is of no significance in phenylketonuric patients on appropriate dietary restriction.
Porphyria and Hypothyroidism
As with all drugs containing sulfonamides, caution is advisable in patients with porphyria or thyroid dysfunction.
Use in the Treatment of and Prophylaxis for Pneumocystis Jiroveci Pneumonia in Patients with Acquired Immunodeficiency Syndrome (AIDS): AIDS patients may not tolerate or respond to sulfamethoxazole and trimethoprim in the same manner as non-AIDS patients. The incidence of side effects, particularly rash, fever, leukopenia and elevated aminotransferase (transaminase) values, with sulfamethoxazole and trimethoprim therapy in AIDS patients who are being treated for Pneumocystis jiroveci pneumonia has been reported to be greatly increased compared with the incidence normally associated with the use of sulfamethoxazole and trimethoprim in non-AIDS patients. The incidence of hyperkalemia appears to be increased in AIDS patients receiving sulfamethoxazole and trimethoprim. Adverse effects are generally less severe in patients receiving sulfamethoxazole and trimethoprim for prophylaxis. A history of mild intolerance to sulfamethoxazole and trimethoprim in AIDS patients does not appear to predict intolerance of subsequent secondary prophylaxis.7 However, if a patient develops skin rash or any sign of adverse reaction, therapy with sulfamethoxazole and trimethoprim should be reevaluated (see WARNINGS ).
Co-administration of sulfamethoxazole and trimethoprim and leucovorin should be avoided with Pneumocystis jiroveci pneumonia (see WARNINGS ).
High dosage of trimethoprim, as used in patients with Pneumocystis jiroveci pneumonia, induces a progressive but reversible increase of serum potassium concentrations in a substantial number of patients. Even treatment with recommended doses may cause hyperkalemia when trimethoprim is administered to patients with underlying disorders of potassium metabolism, with renal insufficiency, or if drugs known to induce hyperkalemia are given concomitantly. Close monitoring of serum potassium is warranted in these patients.
During treatment, adequate fluid intake and urinary output should be ensured to prevent crystalluria. Patients who are “slow acetylators” may be more prone to idiosyncratic reactions to sulfonamides.
Information for Patients: Patients should be counseled that antibacterial drugs including sulfamethoxazole and trimethoprim tablets should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When sulfamethoxazole and trimethoprim tablets are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by sulfamethoxazole and trimethoprim tablets or other antibacterial drugs in the future.
Patients should be instructed to maintain an adequate fluid intake in order to prevent crystalluria and stone formation.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Laboratory Tests: Complete blood counts should be done frequently in patients receiving sulfamethoxazole and trimethoprim; if a significant reduction in the count of any formed blood element is noted, sulfamethoxazole and trimethoprim should be discontinued. Urinalyses with careful microscopic examination and renal function tests should be performed during therapy, particularly for those patients with impaired renal function.
Drug Interactions: In elderly patients concurrently receiving certain diuretics, primarily thiazides, an increased incidence of thrombocytopenia with purpura has been reported.
It has been reported that sulfamethoxazole and trimethoprim may prolong the prothrombin time in patients who are receiving the anticoagulant warfarin. This interaction should be kept in mind when sulfamethoxazole and trimethoprim is given to patients already on anticoagulant therapy, and the coagulation time should be reassessed. Sulfamethoxazole and trimethoprim may inhibit the hepatic metabolism of phenytoin. Sulfamethoxazole and trimethoprim, given at a common clinical dosage, increased the phenytoin half-life by 39% and decreased the phenytoin metabolic clearance rate by 27%. When administering these drugs concurrently, one should be alert for possible excessive phenytoin effect.
Sulfonamides can also displace methotrexate from plasma protein binding sites and can compete with the renal transport of methotrexate, thus increasing free methotrexate concentrations.
There have been reports of marked but reversible nephrotoxicity with coadministration of sulfamethoxazole and trimethoprim and cyclosporine in renal transplant recipients.
Increased digoxin blood levels can occur with concomitant sulfamethoxazole and trimethoprim therapy, especially in elderly patients. Serum digoxin levels should be monitored.
Increased sulfamethoxazole blood levels may occur in patients who are receiving indomethacin.
Occasional reports suggest that patients receiving pyrimethamine as malaria prophylaxis in doses exceeding 25 mg weekly may develop megaloblastic anemia if sulfamethoxazole and trimethoprim is prescribed.
The efficacy of tricyclic antidepressants can decrease when coadministered with sulfamethoxazole and trimethoprim.
Like other sulfonamide-containing drugs, sulfamethoxazole and trimethoprim potentiates the effect of oral hypoglycemics.
In the literature, a single case of toxic delirium has been reported after concomitant intake of trimethoprim/sulfamethoxazole and amantadine.
In the literature, three cases of hyperkalemia in elderly patients have been reported after concomitant intake of trimethoprim/sulfamethoxazole and an angiotensin converting enzyme inhibitor. 8, 9
Drug/Laboratory Test Interactions: Sulfamethoxazole and trimethoprim, specifically the trimethoprim component, can interfere with a serum methotrexate assay as determined by the competitive binding protein technique (CBPA) when a bacterial dihydrofolate reductase is used as the binding protein. No interference occurs, however, if methotrexate is measured by a radioimmunoassay (RIA).
The presence of sulfamethoxazole and trimethoprim may also interfere with the Jaffé alkaline picrate reaction assay for creatinine, resulting in overestimations of about 10% in the range of normal values.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Carcinogenesis: Long-term studies in animals to evaluate carcinogenic potential have not been conducted with sulfamethoxazole and trimethoprim.
Mutagenesis: Bacterial mutagenic studies have not been performed with sulfamethoxazole and trimethoprim in combination. Trimethoprim was demonstrated to be nonmutagenic in the Ames assay. No chromosomal damage was observed in human leukocytes cultured in vitro with sulfamethoxazole and trimethoprim alone or in combination; the concentrations used exceeded blood levels of these compounds following therapy with sulfamethoxazole and trimethoprim. Observations of leukocytes obtained from patients treated with sulfamethoxazole and trimethoprim revealed no chromosomal abnormalities.
Impairment of Fertility: No adverse effects on fertility or general reproductive performance were observed in rats given oral dosages as high as 350 mg/kg/day sulfamethoxazole plus 70 mg/kg/day trimethoprim.
Pregnancy: Teratogenic Effects: Pregnancy Category C. In rats, oral doses of 533 mg/kg or 200 mg/kg produced teratologic effects manifested mainly as cleft palates.
The highest dose which did not cause cleft palates in rats was 512 mg/kg or 192 mg/kg trimethoprim when administered separately. In two studies in rats, no teratology was observed when 512 mg/kg of sulfamethoxazole was used in combination with 128 mg/kg of trimethoprim. In one study, however, cleft palates were observed in one litter out of 9 when 355 mg/kg of sulfamethoxazole was used in combination with 88 mg/kg of trimethoprim.
In some rabbit studies, an overall increase in fetal loss (dead and resorbed and malformed conceptuses) was associated with doses of trimethoprim 6 times the human therapeutic dose.
While there are no large, well-controlled studies on the use of sulfamethoxazole and trimethoprim in pregnant women, Brumfitt and Pursell,10 in a retrospective study, reported the outcome of 186 pregnancies during which the mother received either placebo or sulfamethoxazole and trimethoprim. The incidence of congenital abnormalities was 4.5% (3 of 66) in those who received placebo and 3.3% (4 of 120) in those receiving sulfamethoxazole and trimethoprim. There were no abnormalities in the 10 children whose mothers received the drug during the first trimester. In a separate survey, Brumfitt and Pursell also found no congenital abnormalities in 35 children whose mothers had received oral sulfamethoxazole and trimethoprim at the time of conception or shortly thereafter.
Because sulfamethoxazole and trimethoprim may interfere with folic acid metabolism, sulfamethoxazole and trimethoprim should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects: See CONTRAINDICATIONS section.
Nursing Mothers: See CONTRAINDICATIONS section.
Pediatric Use: Sulfamethoxazole and trimethoprim is not recommended for infants younger than 2 months of age (see INDICATIONS and CONTRAINDICATIONS sections).
Geriatric Use: Clinical studies of sulfamethoxazole and trimethoprim did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
There may be an increased risk of severe adverse reactions in elderly patients, particularly when complicating conditions exist, e.g., impaired kidney and/or liver function, possible folate deficiency, or concomitant use of other drugs. Severe skin reactions, generalized bone marrow suppression (see WARNINGS and ADVERSE REACTIONS sections), a specific decrease in platelets (with or without purpura), and hyperkalemia are the most frequently reported severe adverse reactions in elderly patients. In those concurrently receiving certain diuretics, primarily thiazides, an increased incidence of thrombocytopenia with purpura has been reported. Increased digoxin blood levels can occur with concomitant sulfamethoxazole and trimethoprim therapy, especially in elderly patients. Serum digoxin levels should be monitored. Hematological changes indicative of folic acid deficiency may occur in elderly patients. These effects are reversible by folinic acid therapy. Appropriate dosage adjustments should be made for patients with impaired kidney function and duration of use should be as short as possible to minimize risks of undesired reactions (see DOSAGE AND ADMINISTRATION section). The trimethoprim component of sulfamethoxazole and trimethoprim may cause hyperkalemia when administered to patients with underlying disorders of potassium metabolism, with renal insufficiency, or when given concomitantly with drugs known to induce hyperkalemia, such as angiotensin converting enzyme inhibitors. Close monitoring of serum potassium is warranted in these patients. Discontinuation of sulfamethoxazole and trimethoprim treatment is recommended to help lower potassium serum levels. Sulfamethoxazole and Trimethoprim Tablets contain 1.8 mg sodium (0.08 mEq) of sodium per tablet. Sulfamethoxazole and Trimethoprim Double Strength Tablets contain 3.6 mg (0.16 mEq) of sodium per tablet.
Pharmacokinetics parameters for sulfamethoxazole were similar for geriatric subjects and younger adult subjects. The mean maximum serum trimethoprim concentration was higher and mean renal clearance of trimethoprim was lower in geriatric subjects compared with younger subjects (see CLINICAL PHARMACOLOGY: Geriatric Pharmacokinetics ).
SULFAMETHOXAZOLE AND TRIMETHOPRIM ADVERSE REACTIONS
The most common adverse effects are gastrointestinal disturbances (nausea, vomiting, anorexia) and allergic skin reactions (such as rash and urticaria). FATALITIES ASSOCIATED WITH THE ADMINISTRATION OF SULFONAMIDES, ALTHOUGH RARE, HAVE OCCURRED DUE TO SEVERE REACTIONS, INCLUDING STEVENS-JOHNSON SYNDROME, TOXIC EPIDERMAL NECROLYSIS, FULMINANT HEPATIC NECROSIS, AGRANULOCYTOSIS, APLASTIC ANEMIA AND OTHER BLOOD DYSCRASIAS (SEE WARNINGS SECTION).
Hematologic: Agranulocytosis, aplastic anemia, thrombocytopenia, leukopenia, neutropenia, hemolytic anemia, megaloblastic anemia, hypoprothrombinemia, methemoglobinemia, eosinophilia.
Allergic Reactions: Stevens-Johnson syndrome, toxic epidermal necrolysis, anaphylaxis, allergic myocarditis, erythema multiforme, exfoliative dermatitis, angioedema, drug fever, chills, Henoch-Schoenlein purpura, serum sickness-like syndrome, generalized allergic reactions, generalized skin eruptions, photosensitivity, conjunctival and scleral injection, pruritus, urticaria and rash. In addition, periarteritis nodosa and systemic lupus erythematosus have been reported.
Gastrointestinal: Hepatitis (including cholestatic jaundice and hepatic necrosis), elevation of serum transaminase and bilirubin, pseudomembranous enterocolitis, pancreatitis, stomatitis, glossitis, nausea, emesis, abdominal pain, diarrhea, anorexia.
Genitourinary: Renal failure, interstitial nephritis, BUN and serum creatinine elevation, toxic nephrosis with oliguria and anuria, crystalluria and nephrotoxicity in association with cyclosporine.
Metabolic and Nutritional: Hyperkalemia (see PRECAUTIONS: Use in the Treatment of and Prophylaxis for Pneumocystis Jiroveci Pneumonia in Patients with Acquired Immunodeficiency Syndrome (AIDS) .
Neurologic: Aseptic meningitis, convulsions, peripheral neuritis, ataxia, vertigo, tinnitus, headache.
Psychiatric: Hallucinations, depression, apathy, nervousness.
Endocrine: The sulfonamides bear certain chemical similarities to some goitrogens, diuretics (acetazolamide and the thiazides) and oral hypoglycemic agents. Cross-sensitivity may exist with these agents. Diuresis and hypoglycemia have occurred rarely in patients receiving sulfonamides.
Musculoskeletal: Arthralgia and myalgia. Isolated cases of rhabdomyolysis have been reported with sulfamethoxazole and trimethoprim, mainly in AIDS patients.
Respiratory: Cough, shortness of breath and pulmonary infiltrates (see WARNINGS ).
Miscellaneous: Weakness, fatigue, insomnia.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of trimethoprim-sulfamethoxazole. Because these reactions were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to the drug exposure:
- Thrombotic thrombocytopenia purpura
- Idiopathic thrombocytopenic purpura
OVERDOSAGE
Acute: The amount of a single dose of sulfamethoxazole and trimethoprim that is either associated with symptoms of overdosage or is likely to be life-threatening has not been reported. Signs and symptoms of overdosage reported with sulfonamides include anorexia, colic, nausea, vomiting, dizziness, headache, drowsiness and unconsciousness. Pyrexia, hematuria and crystalluria may be noted. Blood dyscrasias and jaundice are potential late manifestations of overdosage.
Signs of acute overdosage with trimethoprim include nausea, vomiting, dizziness, headache, mental depression, confusion and bone marrow depression.
General principles of treatment include the institution of gastric lavage or emesis, forcing oral fluids and the administration of intravenous fluids if urine output is low and renal function is normal. Acidification of the urine will increase renal elimination of trimethoprim. The patient should be monitored with blood counts and appropriate blood chemistries, including electrolytes. If a significant blood dyscrasia or jaundice occurs, specific therapy should be instituted for these complications. Peritoneal dialysis is not effective and hemodialysis is only moderately effective in eliminating sulfamethoxazole and trimethoprim.
Chronic: Use of sulfamethoxazole and trimethoprim at high doses and/or for extended periods of time may cause bone marrow depression manifested as thrombocytopenia, leukopenia and/or megaloblastic anemia. If signs of bone marrow depression occur, the patient should be given leucovorin 5 to 15 mg daily until normal hematopoiesis is restored.
DOSAGE & ADMINISTRATION
Sulfamethoxazole and trimethoprim tablets, USP are contraindicated in pediatric patients less than 2 months of age.
Urinary Tract Infections and Shigellosis in Adults and Pediatric Patients, and Acute Otitis Media in Children:
Adults: The usual adult dosage in the treatment of urinary tract infections is 1 sulfamethoxazole and trimethoprim DS (double strength) tablet, USP every 12 hours for 10 to 14 days. An identical daily dosage is used for 5 days in the treatment of shigellosis.
Children: The recommended dose for children with urinary tract infections or acute otitis media is 40 mg/kg sulfamethoxazole, USP and 8 mg/kg trimethoprim, USP per 24 hours, given in two divided doses every 12 hours for 10 days. An identical daily dosage is used for 5 days in the treatment of shigellosis. The following table is a guideline for the attainment of this dosage:
| Weight | Dose – every 12 hours | |
| lb | kg | Tablets |
| 22 | 10 | - |
| 44 | 20 | 1 |
| 66 | 30 | 1 ½ |
| 88 | 40 | 2 or 1 DS tablet |
For Patients with Impaired Renal Function
When renal function is impaired, a reduced dosage should be employed using the following table:
| Creatinine Clearance (mL/min) |
Recommended Dosage Regimen |
| Above 30 | Usual standard regimen |
| 15 to 30 | 1/2 the usual regimen |
| Below 15 | Use not recommended |
Acute Exacerbations of Chronic Bronchitis in Adults:
The usual adult dosage in the treatment of acute exacerbations of chronic bronchitis is 1 sulfamethoxazole and trimethoprim double strength tablet, USP, or 2 sulfamethoxazole and trimethoprim single strength tablets, USP, every 12 hours for 14 days.
Pneumocystis JiroveciPneumonia
Treatment: Adults and Children:
The recommended dosage for patients with documented Pneumocystis jiroveci pneumonia is 75 to 100 mg/kg sulfamethoxazole, USP and 15 to 20 mg/kg trimethoprim, USP per 24 hours given in equally divided doses every 6 hours for 14 to 21 days.11 The following table is a guideline for the upper limit of this dosage.
| Weight | Dose – every 6 hours | |
| lb | kg | Tablets |
| 18 | 8 | - |
| 35 | 16 | 1 |
| 53 | 24 | 1 ½ |
| 70 | 32 | 2 or 1 DS tablet |
| 88 | 40 | 2 ½ |
| 106 | 48 | 3 or 1 ½ DS tablets |
| 141 | 64 | 4 or 2 DS tablets |
| 176 | 80 | 5 or 2 ½ DS tablets |
For the lower limit dose (75 mg/kg sulfamethoxazole, USP and 15 mg/kg trimethoprim, USP per 24 hours) administer 75% of the dose in the above table.
Prophylaxis
Adults:
The recommended dosage for prophylaxis in adults is 1 sulfamethoxazole and trimethoprim DS (double strength) tablet, USP daily.12
Children:
For children, the recommended dose is 750 mg/m2/day sulfamethoxazole, USP with 150 mg/m2/day trimethoprim, USP given orally in equally divided doses twice a day, on 3 consecutive days per week.
The total daily dose should not exceed 1600 mg sulfamethoxazole, USP and 320 mg trimethoprim, USP.13 The following table is a guideline for the attainment of this dosage in children:
| Body Surface Area | Dose – every 12 hours |
| (m2) | Tablets |
| 0.26 | - |
| 0.53 | ½ |
| 1.06 | 1 |
Traveler’s Diarrhea in Adults:
For the treatment of traveler’s diarrhea, the usual adult dosage is 1 sulfamethoxazole and trimethoprim DS (double strength) tablet, USP or 2 sulfamethoxazole and trimethoprim single strength tablets, USP every 12 hours for 5 days.
HOW SUPPLIED
Sulfamethoxazole and Trimethoprim Tablets, USP are supplied as follows:
Sulfamethoxazole and Trimethoprim DS (double strength) Tablets, USP, 800 mg/160 mg, are supplied as white, oval, bisected tablets debossed “IP” bisect “272” on one side.
They are available as follows:
Bottles of 10: NDC 53746-272-11
Bottles of 24: NDC 53746-272-24
Bottles of 100: NDC 53746-272-01
Bottles of 250: NDC 53746-272-02
Bottles of 500: NDC 53746-272-05
Sulfamethoxazole and Trimethoprim Tablets, USP, 400 mg/80 mg, are supplied as white, round, bisected tablets debossed “IP” over “271” on one side.
They are available as follows:
Bottles of 50: NDC 53746-271-50
Bottles of 100: NDC 53746-271-01
Bottles of 500: NDC 53746-271-05
Bottles of 1000: NDC 53746-271-10
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
DISPENSE IN TIGHT, LIGHT-RESISTANT CONTAINER.
REFERENCES
- Kremers P, Duvivier J, Heusghem C. Pharmacokinetic Studies of Co-Trimoxazole in Man after Single and Repeated Doses. J Clin Pharmacol. Feb-Mar 1974; 14:112-117.
- Kaplan SA, et al. Pharmacokinetic Profile of Trimethoprim-Sulfamethoxazole in Man. J Infect Dis. Nov 1973; 128 (Suppl): S547-S555.
- Varoquaux O, et al Pharmacokinetics of the trimethoprim-sulfamethoxazole combination in the elderly. Br J Clin Pharmacol. 1985;20;575-581.
- Rudoy RC, Nelson JD, Haltalin KC. Antimicrobial Agents Chemother. May 1974;5:439-443.
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard – Fourth Edition. NCCLS document M7-A4, Vol.17, No. 2 NCCLS, Wayne, PA, January, 1997.
- Safrin S, Lee BL, Sande MA. Adjunctive folinic acid with trimethoprim-sulfamethoxazole for Pneumocystis carinii pneumonia in AIDS patients is associated with an increased risk of therapeutic failure and death. J Infect Dis. 1994 Oct; 170(4):912-7
- Hardy DW, et al. A controlled trial of trimethoprim-sulfamethoxazole or aerosolized pentamidine for secondary prophylaxis of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992; 327:1842-1848.
- Marinella, Mark A. 1999. Trimethoprim-induced hyperkalemia: An analysis of reported cases. Gerontol, 45:209-212.
- Margassery, S. and B. Bastani. 2002. Life threatening hyperkalemia and acidosis secondary to trimethoprim-sulfamethoxazole treatment. J. Nephrol. 14:410-414.
- Brumfitt W, Pursell R. Trimethoprim/Sulfamethoxazole in the Treatment of Bacteriuria in Women. J Infect Dis. Nov 1973; 128 (Suppl):S657-S663.
- Masur H. Prevention and treatment of Pneumocystis pneumonia. N Engl J Med. 1992; 327:1853-1880.
- Recommendations for prophylaxis against Pneumocystis carinii pneumonia for adults and adolescents infected with human immunodeficiency virus. MMWR. 1992; 41(RR-4):1-11.
- CDC Guidelines for prophylaxis against Pneumocystis carinii pneumonia for children infected with human immunodeficiency virus. MMWR. 1991; 40(RR-2):1-13.
SPL UNCLASSIFIED
Manufactured by:
Amneal Pharmaceuticals of NY
Hauppauge, NY 11788
Distributed by:
Amneal Pharmaceuticals
Glasgow, KY 42141
Rev. 01-2013
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
DRUG: Sulfamethoxazole and Trimethoprim
GENERIC: Sulfamethoxazole and Trimethoprim
DOSAGE: TABLET
ADMINSTRATION: ORAL
NDC: 24236-577-20
ACTIVE INGREDIENT(S):
- SULFAMETHOXAZOLE 800mg in 1
- TRIMETHOPRIM 160mg in 1
INACTIVE INGREDIENT(S):
- MAGNESIUM STEARATE
- STARCH, CORN
- POVIDONE
- SODIUM STARCH GLYCOLATE TYPE A POTATO
COLOR: white
SHAPE: OVAL
SCORE: Two even pieces
SIZE: 19 mm
IMPRINT: IP;272
PACKAGING: 100 in 1 VIAL


Sulfamethoxazole and TrimethoprimSulfamethoxazole and Trimethoprim TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||