Sulfasalazine
FULL PRESCRIBING INFORMATION: CONTENTS*
- SULFASALAZINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- SULFASALAZINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- SULFASALAZINE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
SULFASALAZINE DESCRIPTION
Therapeutic Classification:
Chemical Designation:
Structural Formula:
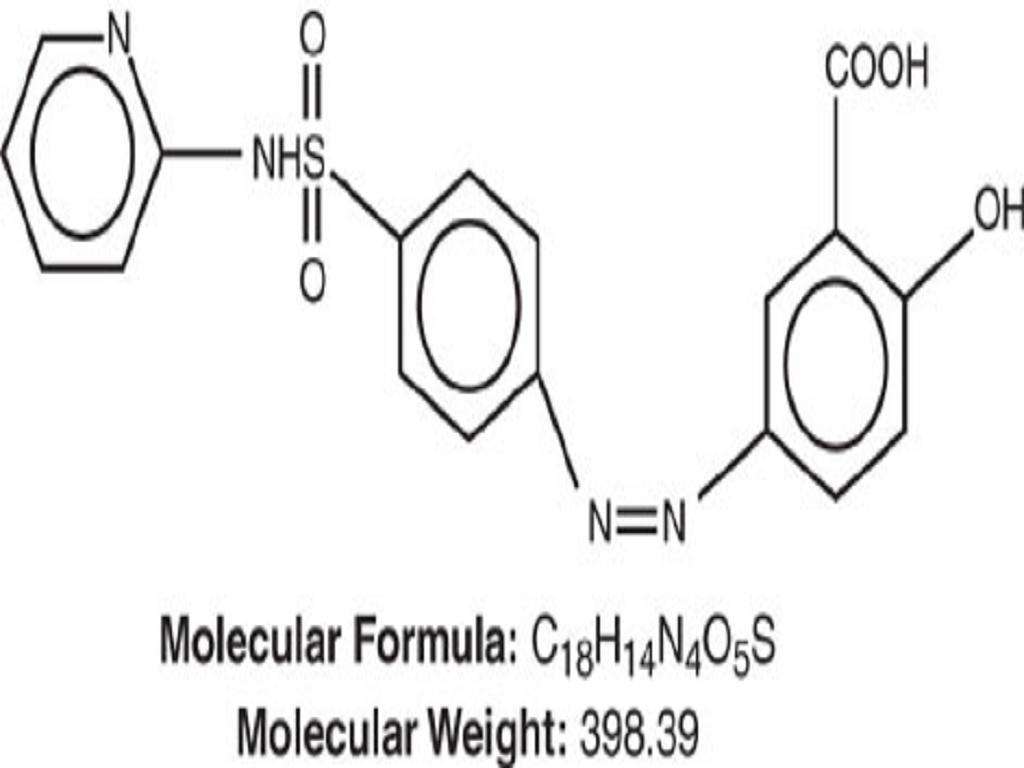
Inactive ingredients:
CLINICAL PHARMACOLOGY
Pharmacodynamics
Pharmacokinetics
Absorption:
Distribution:
Metabolism:
Excretion:
Special Populations
Elderly:
Pediatric:
Acetylator Status:
Gender:
INDICATIONS & USAGE
Sulfasalazine tablets, USP are indicated:SULFASALAZINE CONTRAINDICATIONS
-
● Patients with intestinal or urinary obstruction,
-
● Patients with porphyria as sulfonamides have been reported to precipitate an acute attack,
-
● Patients hypersensitive to sulfasalazine, its metabolites, sulfonamides, or salicylates.
WARNINGS
PRECAUTIONS, Laboratory TestsPRECAUTIONS
General:INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects:Pregnancy Category B:
Nonteratogenic Effects:
NURSING MOTHERS
PEDIATRIC USE
SULFASALAZINE ADVERSE REACTIONS
Blood dyscrasias:
Hypersensitivity reactions:
Gastrointestinal reactions:
Central nervous system reactions:
Renal reactions:
Other reactions:
Postmarketing Reports
Gastrointestinal:
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
Instructions for overdosage:
DOSAGE & ADMINISTRATION
Initial Therapy:
Adults:
Children, six years of age and older:
Maintenance Therapy:
Adults:
Children, six years of age and older:
HOW SUPPLIED
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

SulfasalazineSulfasalazine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!