Sumatriptan
FULL PRESCRIBING INFORMATION: CONTENTS*
- SUMATRIPTAN DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- INDICATIONS & USAGE
- SUMATRIPTAN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- SUMATRIPTAN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- MEDICATION GUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
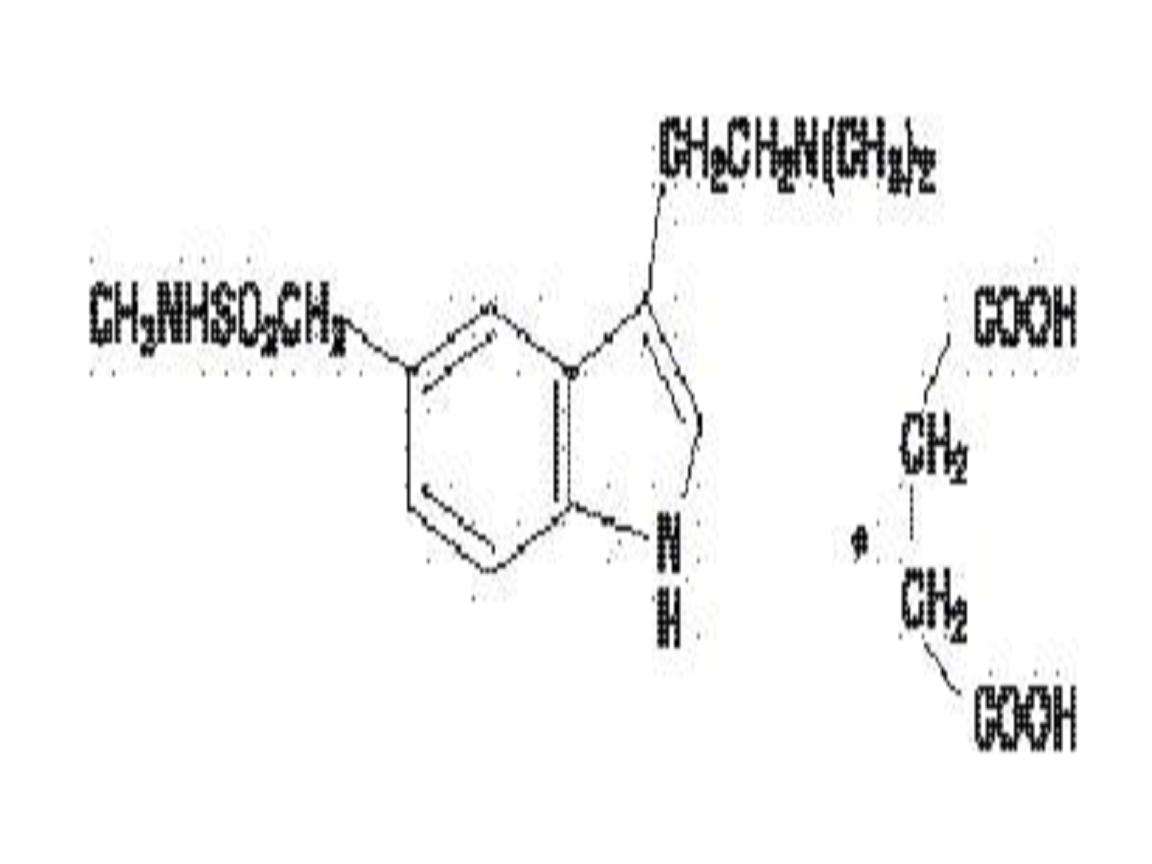
SUMATRIPTAN DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of ActionPharmacokinetics
Special Populations
Renal Impairment
Hepatic Impairment
Age
Gender
Race
Drug Interactions
Monoamine Oxidase Inhibitors
Alcohol
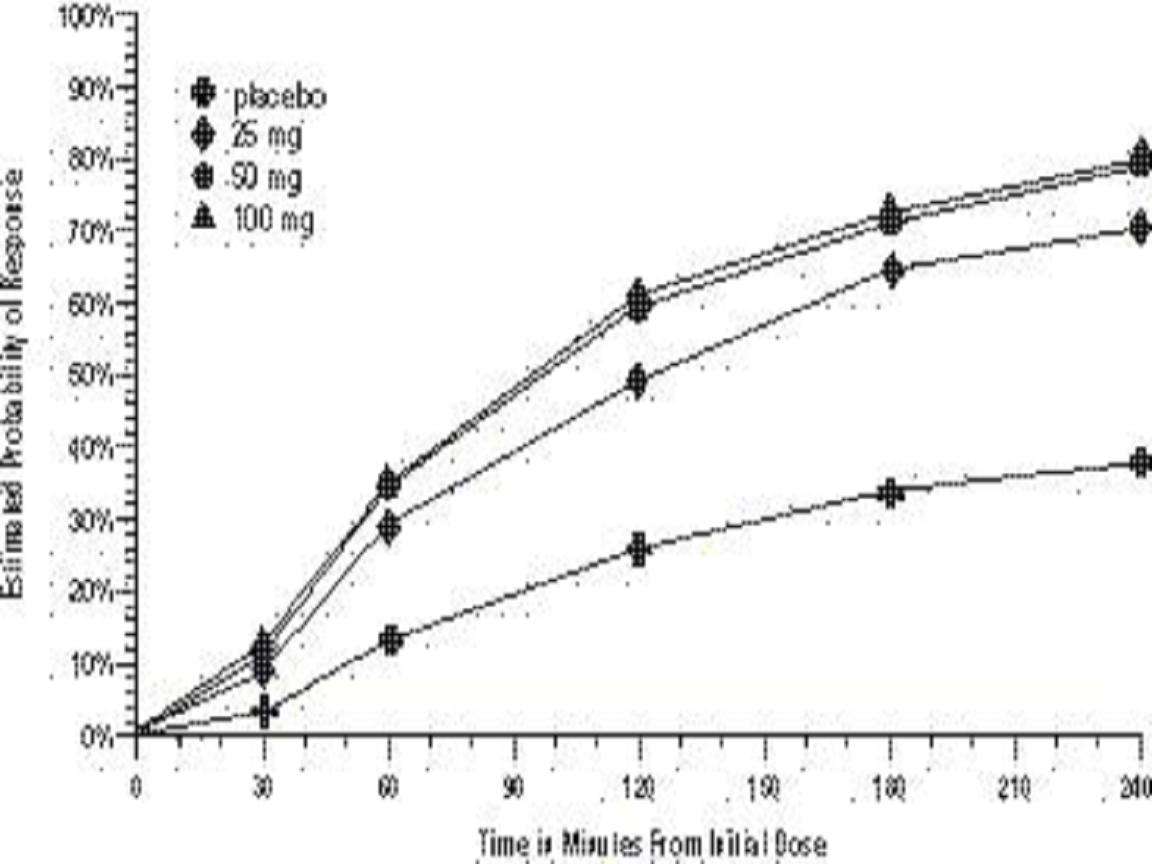
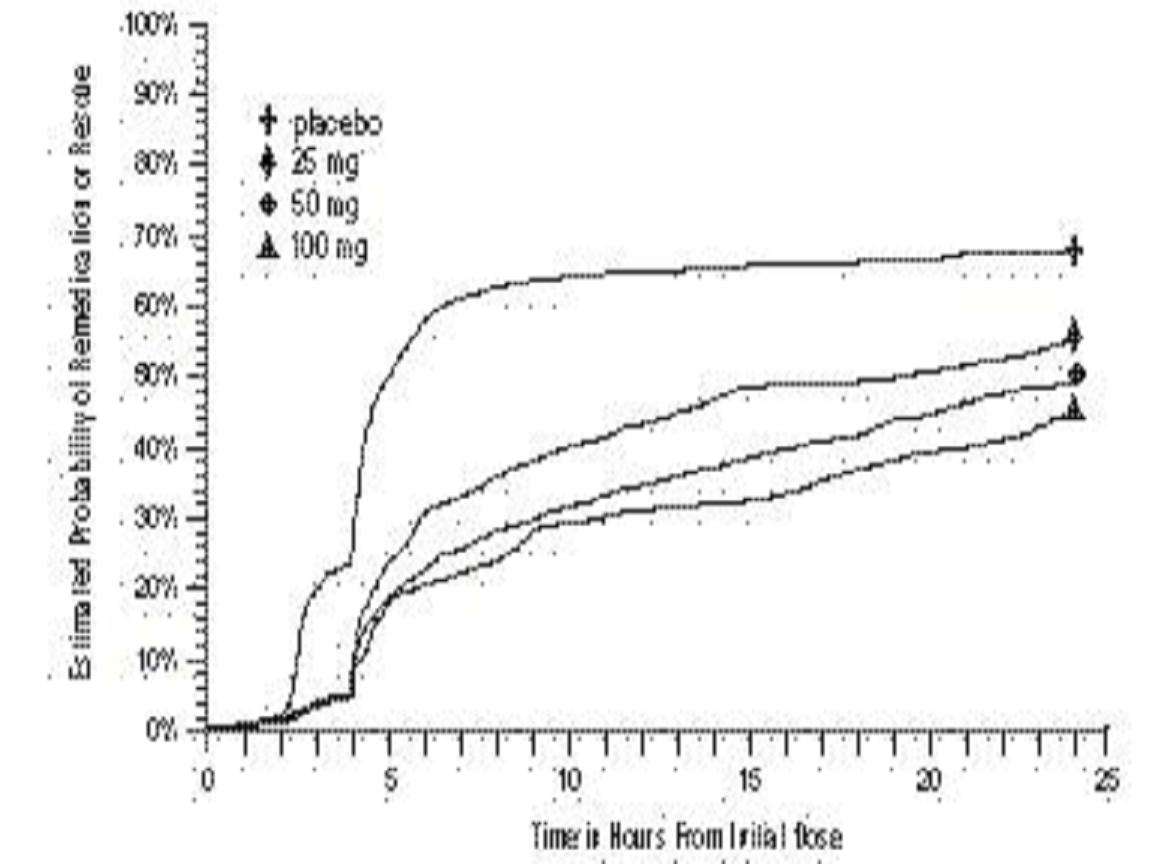
CLINICAL STUDIES
Comparisons of drug performance based upon results obtained in different clinical trials are never reliable. Because studies are conducted at different times, with different samples of patients, by different investigators, employing different criteria and/or different interpretations of the same criteria, under different conditions (dose, dosing regimen, etc.), quantitative estimates of treatment response and the timing of response may be expected to vary considerably from study to study.
INDICATIONS & USAGE
SUMATRIPTAN CONTRAINDICATIONS
Sumatriptan tablets should not be given to patients with history, symptoms, or signs of ischemic cardiac, cerebrovascular, or peripheral vascular syndromes. In addition, patients with other significant underlying cardiovascular diseases should not receive sumatriptan tablets. Ischemic cardiac syndromes include, but are not limited to, angina pectoris of any type (e.g., stable angina of effort and vasospastic forms of angina such as the Prinzmetal variant), all forms of myocardial infarction, and silent myocardial ischemia.Cerebrovascular syndromes include, but are not limited to, strokes of any type as well as transient ischemic attacks. Peripheral vascular disease includes, but is not limited to, ischemic bowel disease (seeWARNINGS).
Because sumatriptan tablets may increase blood pressure, they should not be given to patients with uncontrolled hypertension.
Concurrent administration of MAO-A inhibitors or use within 2 weeks of discontinuation of MAO-A inhibitor therapy is contraindicated (seeCLINICAL PHARMACOLOGY: Drug InteractionsandPRECAUTIONS: Drug Interactions).
Sumatriptan tablets should not be administered to patients with hemiplegic or basilar migraine.
Sumatriptan tablets and any ergotamine-containing or ergot-type medication (like dihydroergotamine or methysergide) should not be used within 24 hours of each other, nor should sumatriptan and another 5-HT1agonist.
Sumatriptan tablets are contraindicated in patients with hypersensitivity to sumatriptan or any of their components.
Sumatriptan tablets are contraindicated in patients with severe hepatic impairment.
WARNINGS
Sumatriptan tablets should only be used where a clear diagnosis of migraine headache has been established.Risk of Myocardial Ischemia and/or Infarction and Other Adverse Cardiac Events
Sumatriptan should not be given to patients with documented ischemic or vasospastic coronary artery disease (CAD) (seeCONTRAINDICATIONS). It is strongly recommended that sumatriptan not be given to patients in whom unrecognized CAD is predicted by the presence of risk factors (e.g., hypertension, hypercholesterolemia, smoker, obesity, diabetes, strong family history of CAD, female with surgical or physiological menopause, or male over 40 years of age) unless a cardiovascular evaluation provides satisfactory clinical evidence that the patient is reasonably free of coronary artery and ischemic myocardial disease or other significant underlying cardiovascular disease. The sensitivity of cardiac diagnostic procedures to detect cardiovascular disease or predisposition to coronary artery vasospasm is modest, at best. If, during the cardiovascular evaluation, the patient's medical history or electrocardiographic investigations reveal findings indicative of, or consistent with, coronary artery vasospasm or myocardial ischemia, sumatriptan should not be administered (seeCONTRAINDICATIONS).
For patients with risk factors predictive of CAD, who are determined to have a satisfactory cardiovascular evaluation, it is strongly recommended that administration of the first dose of sumatriptan tablets take place in the setting of a physician's office or similar medically staffed and equipped facility unless the patient has previously received sumatriptan. Because cardiac ischemia can occur in the absence of clinical symptoms, consideration should be given to obtaining on the first occasion of use an electrocardiogram (ECG) during the interval immediately following sumatriptan tablets, in these patients with risk factors.
It is recommended that patients who are intermittent long-term users of sumatriptan and who have or acquire risk factors predictive of CAD, as described above, undergo periodic interval cardiovascular evaluation as they continue to use sumatriptan.
The systematic approach described above is intended to reduce the likelihood that patients with unrecognized cardiovascular disease will be inadvertently exposed to sumatriptan.
Drug-Associated Cardiac Events and Fatalities
Premarketing Experience With Sumatriptan
Postmarketing Experience With Sumatriptan
Drug-Associated Cerebrovascular Events and Fatalities
Other Vasospasm-Related Events
Serotonin Syndrome
Increase in Blood Pressure
Concomitant Drug Use
Hypersensitivity
PRECAUTIONS
GeneralBinding to Melanin-Containing Tissues
Corneal Opacities
INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
Selective Serotonin Reuptake Inhibitors/Serotonin Norepinephrine Reuptake Inhibitors and Serotonin SyndromeErgot-Containing Drugs
Monoamine Oxidase-A Inhibitors
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CarcinogenesisMutagenesis
Impairment of Fertility
PREGNANCY
Embryolethality
Teratogenicity
Pup Deaths
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
SUMATRIPTAN ADVERSE REACTIONS
Serious cardiac events, including some that have been fatal, have occurred following the use of sumatriptan succinate injection or tablets. These events are extremely rare and most have been reported in patients with risk factors predictive of CAD. Events reported have included coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, and ventricular fibrillation(seeCONTRAINDICATIONS, WARNINGS, and PRECAUTIONS).Incidence in Controlled Clinical Trials
Other Events Observed in Association With the Administration of Sumatriptan Tablets
Atypical Sensations
Cardiovascular
Ear, Nose, and Throat
Endocrine and Metabolic
Eye
Gastrointestinal
Hematological Disorders
Musculoskeletal
Neurological
Respiratory
Skin
Breasts
Urogenital
Miscellaneous
Other Events Observed in the Clinical Development of Sumatriptan
Atypical Sensations
Cardiovascular
Chest Symptoms
Endocrine and Metabolic
Ear, Nose, and Throat
Eye
Gastrointestinal
Injection Site Reaction
Miscellaneous
Mouth and Teeth
Musculoskeletal
Neurological
Respiratory
Skin
Urogenital
Postmarketing Experience (Reports for Subcutaneous or Oral Sumatriptan)
Blood
Cardiovascular
Ear, Nose, and Throat
Eye
Gastrointestinal
Hepatic
Neurological
Non-Site Specific
Psychiatry
Respiratory
Skin
Urogenital
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
DOSAGE & ADMINISTRATION
HOW SUPPLIED
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
Corneal OpacitiesMEDICATION GUIDE
SUMATRIPTAN TABLETS, USPRx only
Information About Your Medicine:
1. The Purpose of Your Medicine:
2. Important Questions to Consider Before Taking Sumatriptan Tablets, USP:
-
● Are you pregnant? Do you think you might be pregnant? Are you trying to become pregnant? Are you using inadequate contraception? Are you breastfeeding?
-
● Do you have any chest pain, heart disease, shortness of breath, or irregular heartbeats? Have you had a heart attack?
-
● Do you have risk factors for heart disease (such as high blood pressure, high cholesterol, obesity, diabetes, smoking, strong family history of heart disease, or you are postmenopausal or a male over 40 years of age)?
-
● Have you had a stroke, transient ischemic attacks (TIAs), or Raynaud syndrome?
-
● Do you have high blood pressure?
-
● Have you ever had to stop taking this or any other medicine because of an allergy or other problems?
-
● Are you taking any other migraine medicines, including other 5-HT1 agonists or any other medicines containing ergotamine, dihydroergotamine, or methysergide?
-
● Are you taking any medicine for depression or other disorders such as monoamine oxidase inhibitors, selective serotonin reuptake inhibitors (SSRIs), or serotonin norepinephrine reuptake inhibitors (SNRIs)? Common SSRIs are citalopram HBr (CELEXAescitalopram oxalate (LEXAPROparoxetine (PAXILfluoxetine (PROZAColanzapine/fluoxetine (SYMBYAXsertraline (ZOLOFTand fluvoxamine. Common SNRIs are duloxetine (CYMBALTAand venlafaxine (EFFEXOR
-
● Have you had, or do you have, any disease of the liver or kidney?
-
● Have you had, or do you have, epilepsy or seizures?
-
● Is this headache different from your usual migraine attacks?
3. The Use of Sumatriptan Tablets, USP During Pregnancy:
4. How to Use Sumatriptan Tablets, USP:
5. Side Effects to Watch for:
-
● Some patients experience pain or tightness in the chest or throat when using sumatriptan tablets, USP. If this happens to you, then discuss it with your doctor before using any more sumatriptan tablets, USP. If the chest pain is severe or does not go away, call your doctor immediately.
-
● If you have sudden and/or severe abdominal pain following sumatriptan tablets, USP, call your doctor immediately.
-
● Some people may have a reaction called serotonin syndrome when they use certain types of antidepressants, SSRIs or SNRIs, while taking sumatriptan tablets, USP. Symptoms may include confusion, hallucinations, fast heartbeat, feeling faint, fever, sweating, muscle spasm, difficulty walking, and/or diarrhea. Call your doctor immediately if you have any of these symptoms after taking sumatriptan tablets, USP.
-
● Shortness of breath; wheeziness; heart throbbing; swelling of eyelids, face, or lips; or a skin rash, skin lumps, or hives happens rarely. If it happens to you, then tell your doctor immediately. Do not take any more sumatriptan tablets, USP unless your doctor tells you to do so.
-
● Some people may have feelings of tingling, heat, flushing (redness of face lasting a short time), heaviness or pressure after treatment with sumatriptan tablets, USP. A few people may feel drowsy, dizzy, tired, or sick. Tell your doctor of these symptoms at your next visit.
-
● If you feel unwell in any other way or have any symptoms that you do not understand, you should contact your doctor immediately.
7. Storing Your Medicine:
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

SumatriptanSumatriptan Succinate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!