SUTENT
Pfizer Laboratories Div Pfizer Inc
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SUTENT safely and effectively. See full prescribing information for SUTENT. SUTENT (sunitinib malate) capsules, oralInitial U.S. Approval: 2006BOXED WARNINGWARNING: HEPATOTOXICITY See full prescribing information for complete boxed warning. Hepatotoxicity has been observed in clinical trials and post-marketing experience. This hepatotoxicity may be severe, and deaths have been reported. [See Warnings and Precautions (5.1)] RECENT MAJOR CHANGES Warnings and Precautions, Thyroid Dysfunction (5.9) 08/2013 INDICATIONS AND USAGESUTENT is a kinase inhibitor indicated for the treatment of: Gastrointestinal stromal tumor (GIST) after disease progression on or intolerance to imatinib mesylate. (1.1) Advanced renal cell carcinoma (RCC). (1.2) Progressive, well-differentiated pancreatic neuroendocrine tumors (pNET) in patients with unresectable locally advanced or metastatic disease. (1.3) DOSAGE AND ADMINISTRATIONGIST and RCC: 50 mg orally once daily, with or without food, 4 weeks on treatment followed by 2 weeks off. (2.1) pNET: 37.5 mg orally once daily, with or without food, continuously without a scheduled off-treatment period. (2.2) Dose Modification: Dose interruptions and/or dose adjustments of 12.5 mg recommended based on individual safety and tolerability. (2.3) DOSAGE FORMS AND STRENGTHS Capsules: 12.5 mg, 25 mg, 50 mg (3) CONTRAINDICATIONS None (4) WARNINGS AND PRECAUTIONS Hepatotoxicity, including liver failure, has been observed. Monitor liver function tests before initiation of treatment, during each cycle of treatment, and as clinically indicated. SUTENT should be interrupted for Grade 3 or 4 drug-related hepatic adverse events and discontinued if there is no resolution. Do not restart SUTENT if patients subsequently experience severe changes in liver function tests or have other signs and symptoms of liver failure. (5.1) Women of childbearing potential should be advised of the potential hazard to the fetus and to avoid becoming pregnant. (5.2) Cardiac toxicity including left ventricular ejection fraction declines to below the lower limit of normal and cardiac failure including death have occurred. Monitor patients for signs and symptoms of congestive heart failure. (5.3) Prolonged QT intervals and Torsade de Pointes have been observed. Use with caution in patients at higher risk for developing QT interval prolongation. When using SUTENT, monitoring with on-treatment electrocardiograms and electrolytes should be considered. (5.4) Hypertension may occur. Monitor blood pressure and treat as needed. (5.5) Hemorrhagic events including tumor-related hemorrhage have occurred. Perform serial complete blood counts and physical examinations. (5.6) Osteonecrosis of the jaw has been reported. Consider preventive dentistry prior to treatment with SUTENT. If possible, avoid invasive dental procedures, particularly in patients receiving intravenous bisphosphonate therapy. (5.7) Cases of Tumor Lysis Syndrome (TLS) have been reported primarily in patients with RCC and GIST with high tumor burden. Monitor these patients closely and treat as clinically indicated. (5.8) Thyroid dysfunction may occur. Patients with signs and/or symptoms suggestive of hypothyroidism or hyperthyroidism should have laboratory monitoring of thyroid function performed and be treated as per standard medical practice. (5.9) Temporary interruption of therapy with SUTENT is recommended in patients undergoing major surgical procedures. (5.10) Adrenal hemorrhage was observed in animal studies. Monitor adrenal function in case of stress such as surgery, trauma or severe infection. (5.11) Side Effects The most common adverse reactions (≥20%) are fatigue, asthenia, fever, diarrhea, nausea, mucositis/stomatitis, vomiting, dyspepsia, abdominal pain, constipation, hypertension, peripheral edema, rash, hand-foot syndrome, skin discoloration, dry skin, hair color changes, altered taste, headache, back pain, arthralgia, extremity pain, cough, dyspnea, anorexia, and bleeding. (6) To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS CYP3A4 Inhibitors: Consider dose reduction of SUTENT when administered with strong CYP3A4 inhibitors. (7.1) CYP3A4 Inducers: Consider dose increase of SUTENT when administered with CYP3A4 inducers. (7.2)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 SUTENT INDICATIONS AND USAGE
- 2 SUTENT DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 SUTENT CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 5.1 Hepatotoxicity
- 5.2 Pregnancy
- 5.3 Left Ventricular Dysfunction
- 5.4 QT Interval Prolongation and Torsade de Pointes
- 5.5 Hypertension
- 5.6 Hemorrhagic Events
- 5.7 Osteonecrosis of the Jaw (ONJ)
- 5.8 Tumor Lysis Syndrome (TLS)
- 5.9 Thyroid Dysfunction
- 5.10 Wound Healing
- 5.11 Adrenal Function
- 5.12 Laboratory Tests
- 6 SUTENT ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 SUTENT DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
Hepatotoxicity has been observed in clinical trials and post-marketing experience. This hepatotoxicity may be severe, and deaths have been reported. [See Warnings and Precautions (5.1)]
1 INDICATIONS AND USAGE
1.1 Gastrointestinal Stromal Tumor (GIST)
SUTENT is indicated for the treatment of gastrointestinal stromal tumor after disease progression on or intolerance to imatinib mesylate.
1.2 Advanced Renal Cell Carcinoma (RCC)
SUTENT is indicated for the treatment of advanced renal cell carcinoma.
1.3 Advanced Pancreatic Neuroendocrine Tumors (pNET)
SUTENT is indicated for the treatment of progressive, well-differentiated pancreatic neuroendocrine tumors in patients with unresectable locally advanced or metastatic disease.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose for GIST and RCC
The recommended dose of SUTENT for gastrointestinal stromal tumor (GIST) and advanced renal cell carcinoma (RCC) is one 50 mg oral dose taken once daily, on a schedule of 4 weeks on treatment followed by 2 weeks off (Schedule 4/2). SUTENT may be taken with or without food.
2.2 Recommended Dose for pNET
The recommended dose of SUTENT for pancreatic neuroendocrine tumors (pNET) is 37.5 mg taken orally once daily continuously without a scheduled off-treatment period. SUTENT may be taken with or without food.
2.3 Dose Modification
Dose interruption and/or dose modification in 12.5 mg increments or decrements is recommended based on individual safety and tolerability. The maximum dose administered in the Phase 3 pNET study was 50 mg daily.
Strong CYP3A4 inhibitors such as ketoconazole may increase sunitinib plasma concentrations. Selection of an alternate concomitant medication with no or minimal enzyme inhibition potential is recommended. A dose reduction for SUTENT to a minimum of 37.5 mg (GIST and RCC) or 25 mg (pNET) daily should be considered if SUTENT must be co-administered with a strong CYP3A4 inhibitor [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
CYP3A4 inducers such as rifampin may decrease sunitinib plasma concentrations. Selection of an alternate concomitant medication with no or minimal enzyme induction potential is recommended. A dose increase for SUTENT to a maximum of 87.5 mg (GIST and RCC) or 62.5 mg (pNET) daily should be considered if SUTENT must be co-administered with a CYP3A4 inducer. If dose is increased, the patient should be monitored carefully for toxicity [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
12.5 mg capsules
Hard gelatin capsule with orange cap and orange body, printed with white ink "Pfizer" on the cap and "STN 12.5 mg" on the body.
25 mg capsules
Hard gelatin capsule with caramel cap and orange body, printed with white ink "Pfizer" on the cap and "STN 25 mg" on the body.
50 mg capsules
Hard gelatin capsule with caramel top and caramel body, printed with white ink "Pfizer" on the cap and "STN 50 mg" on the body.
4 CONTRAINDICATIONS
None
5 WARNINGS AND PRECAUTIONS
5.1 Hepatotoxicity
SUTENT has been associated with hepatotoxicity, which may result in liver failure or death. Liver failure has been observed in clinical trials (7/2281 [0.3%]) and post-marketing experience. Liver failure signs include jaundice, elevated transaminases and/or hyperbilirubinemia in conjunction with encephalopathy, coagulopathy, and/or renal failure. Monitor liver function tests (ALT, AST, bilirubin) before initiation of treatment, during each cycle of treatment, and as clinically indicated. SUTENT should be interrupted for Grade 3 or 4 drug-related hepatic adverse events and discontinued if there is no resolution. Do not restart SUTENT if patients subsequently experience severe changes in liver function tests or have other signs and symptoms of liver failure.
Safety in patients with ALT or AST >2.5 × ULN or, if due to liver metastases, >5.0 × ULN has not been established.
5.2 Pregnancy
SUTENT can cause fetal harm when administered to a pregnant woman. As angiogenesis is a critical component of embryonic and fetal development, inhibition of angiogenesis following administration of SUTENT should be expected to result in adverse effects on pregnancy. In animal reproductive studies in rats and rabbits, sunitinib was teratogenic, embryotoxic, and fetotoxic. There are no adequate and well-controlled studies of SUTENT in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus. Women of childbearing potential should be advised to avoid becoming pregnant while receiving treatment with SUTENT.
5.3 Left Ventricular Dysfunction
In the presence of clinical manifestations of congestive heart failure (CHF), discontinuation of SUTENT is recommended. The dose of SUTENT should be interrupted and/or reduced in patients without clinical evidence of CHF but with an ejection fraction <50% and >20% below baseline.
Cardiovascular events, including heart failure, myocardial disorders and cardiomyopathy, some of which were fatal, have been reported through post-marketing experience. For GIST and RCC, more patients treated with SUTENT experienced decline in left ventricular ejection fraction (LVEF) than patients receiving either placebo or interferon-α (IFN-α). In the double-blind treatment phase of GIST Study A, 22/209 patients (11%) on SUTENT and 3/102 patients (3%) on placebo had treatment-emergent LVEF values below the lower limit of normal (LLN). Nine of 22 GIST patients on SUTENT with LVEF changes recovered without intervention. Five patients had documented LVEF recovery following intervention (dose reduction: one patient; addition of antihypertensive or diuretic medications: four patients). Six patients went off study without documented recovery. Additionally, three patients on SUTENT had Grade 3 reductions in left ventricular systolic function to LVEF <40%; two of these patients died without receiving further study drug. No GIST patients on placebo had Grade 3 decreased LVEF. In the double-blind treatment phase of GIST Study A, 1 patient on SUTENT and 1 patient on placebo died of diagnosed heart failure; 2 patients on SUTENT and 2 patients on placebo died of treatment-emergent cardiac arrest.
In the treatment-naïve RCC study, 103/375 (27%) and 54/360 (15%) patients on SUTENT and IFN-α, respectively, had an LVEF value below the LLN. Twenty-six patients on SUTENT (7%) and seven on IFN-α (2%) experienced declines in LVEF to >20% below baseline and to below 50%. Left ventricular dysfunction was reported in four patients (1%) and CHF in two patients (<1%) who received SUTENT.
In the Phase 3 pNET study, cardiac failure leading to death was reported in 2/83 (2%) patients on SUTENT and no patients on placebo.
Patients who presented with cardiac events within 12 months prior to SUTENT administration, such as myocardial infarction (including severe/unstable angina), coronary/peripheral artery bypass graft, symptomatic CHF, cerebrovascular accident or transient ischemic attack, or pulmonary embolism were excluded from SUTENT clinical studies. It is unknown whether patients with these concomitant conditions may be at a higher risk of developing drug-related left ventricular dysfunction. Physicians are advised to weigh this risk against the potential benefits of the drug. These patients should be carefully monitored for clinical signs and symptoms of CHF while receiving SUTENT. Baseline and periodic evaluations of LVEF should also be considered while these patients are receiving SUTENT. In patients without cardiac risk factors, a baseline evaluation of ejection fraction should be considered.
5.4 QT Interval Prolongation and Torsade de Pointes
SUTENT has been shown to prolong the QT interval in a dose dependent manner, which may lead to an increased risk for ventricular arrhythmias including Torsade de Pointes. Torsade de Pointes has been observed in <0.1% of SUTENT-exposed patients.
SUTENT should be used with caution in patients with a history of QT interval prolongation, patients who are taking antiarrhythmics, or patients with relevant pre-existing cardiac disease, bradycardia, or electrolyte disturbances. When using SUTENT, periodic monitoring with on-treatment electrocardiograms and electrolytes (magnesium, potassium) should be considered. Concomitant treatment with strong CYP3A4 inhibitors, which may increase sunitinib plasma concentrations, should be used with caution and dose reduction of SUTENT should be considered [see Dosage and Administration (2.2)].
5.5 Hypertension
Patients should be monitored for hypertension and treated as needed with standard anti-hypertensive therapy. In cases of severe hypertension, temporary suspension of SUTENT is recommended until hypertension is controlled.
Of patients receiving SUTENT for treatment-naïve RCC, 127/375 patients (34%) receiving SUTENT compared with 13/360 patients (4%) on IFN-α experienced hypertension. Grade 3 hypertension was observed in 50/375 treatment-naïve RCC patients (13%) on SUTENT compared to 1/360 patients (<1%) on IFN-α. While all-grade hypertension was similar in GIST patients on SUTENT compared to placebo, Grade 3 hypertension was reported in 9/202 GIST patients on SUTENT (4%), and none of the GIST patients on placebo. Of patients receiving SUTENT in the Phase 3 pNET study, 22/83 patients (27%) on SUTENT and 4/82 patients (5%) on placebo experienced hypertension. Grade 3 hypertension was reported in 8/83 pNET patients (10%) on SUTENT, and 1/82 patient (1%) on placebo. No Grade 4 hypertension was reported. SUTENT dosing was reduced or temporarily delayed for hypertension in 21/375 patients (6%) on the treatment-naive RCC study and 7/83 pNET patients (8%). Four treatment-naïve RCC patients, including one with malignant hypertension, one patient with pNET, and no GIST patients discontinued treatment due to hypertension. Severe hypertension (>200 mmHg systolic or 110 mmHg diastolic) occurred in 8/202 GIST patients on SUTENT (4%), 1/102 GIST patients on placebo (1%), in 32/375 treatment-naïve RCC patients (9%) on SUTENT, in 3/360 patients (1%) on IFN-α, and in 8/80 pNET patients (10%) on SUTENT and 2/76 pNET patients (3%) on placebo.
5.6 Hemorrhagic Events
Hemorrhagic events reported through post-marketing experience, some of which were fatal, have included GI, respiratory, tumor, urinary tract and brain hemorrhages. In patients receiving SUTENT in a clinical trial for treatment-naïve RCC, 140/375 patients (37%) had bleeding events compared with 35/360 patients (10%) receiving IFN-α. Bleeding events occurred in 37/202 patients (18%) receiving SUTENT in the double-blind treatment phase of GIST Study A, compared to 17/102 patients (17%) receiving placebo. Epistaxis was the most common hemorrhagic adverse event reported. Bleeding events, excluding epistaxis, occurred in 18/83 patients (22%) receiving SUTENT in the Phase 3 pNET study, compared to 8/82 patients (10%) receiving placebo. Epistaxis was reported in 17/83 patients (20%) receiving SUTENT for pNET and 4 patients (5%) receiving placebo. Less common bleeding events in GIST, RCC and pNET patients included rectal, gingival, upper gastrointestinal, genital, and wound bleeding. In the double-blind treatment phase of GIST Study A, 14/202 patients (7%) receiving SUTENT and 9/102 patients (9%) on placebo had Grade 3 or 4 bleeding events. In addition, one patient in GIST Study A taking placebo had a fatal gastrointestinal bleeding event during Cycle 2. Most events in RCC patients were Grade 1 or 2; there was one Grade 5 event of gastric bleed in a treatment-naïve patient. In the pNET study, 1/83 patients (1%) receiving SUTENT had Grade 3 epistaxis, and no patients had other Grade 3 or 4 bleeding events. In pNET patients receiving placebo, 3/82 patients (4%) had Grade 3 or 4 bleeding events.
Tumor-related hemorrhage has been observed in patients treated with SUTENT. These events may occur suddenly, and in the case of pulmonary tumors may present as severe and life-threatening hemoptysis or pulmonary hemorrhage. Cases of pulmonary hemorrhage, some with a fatal outcome, have been observed in clinical trials and have been reported in post-marketing experience in patients treated with SUTENT for MRCC, GIST and metastatic lung cancer. SUTENT is not approved for use in patients with lung cancer. Treatment-emergent Grade 3 and 4 tumor hemorrhage occurred in 5/202 patients (3%) with GIST receiving SUTENT on Study A. Tumor hemorrhages were observed as early as Cycle 1 and as late as Cycle 6. One of these five patients received no further drug following tumor hemorrhage. None of the other four patients discontinued treatment or experienced dose delay due to tumor hemorrhage. No patients with GIST in the Study A placebo arm were observed to undergo intratumoral hemorrhage. Clinical assessment of these events should include serial complete blood counts (CBCs) and physical examinations.
Serious, sometimes fatal gastrointestinal complications including gastrointestinal perforation, have occurred rarely in patients with intra-abdominal malignancies treated with SUTENT.
5.7 Osteonecrosis of the Jaw (ONJ)
ONJ has been observed in clinical trials and has been reported in post-marketing experience in patients treated with sunitinib. Concomitant exposure to other risk factors, such as bisphosphonates or dental disease, may increase the risk of osteonecrosis of the jaw.
5.8 Tumor Lysis Syndrome (TLS)
Cases of TLS, some fatal, have been observed in clinical trials and have been reported in post-marketing experience, primarily in patients with RCC or GIST treated with SUTENT. Patients generally at risk of TLS are those with high tumor burden prior to treatment. These patients should be monitored closely and treated as clinically indicated.
5.9 Thyroid Dysfunction
Baseline laboratory measurement of thyroid function is recommended and patients with hypothyroidism or hyperthyroidism should be treated as per standard medical practice prior to the start of SUTENT treatment. All patients should be observed closely for signs and symptoms of thyroid dysfunction, including hypothyroidism, hyperthyroidism, and thyroiditis, on SUTENT treatment. Patients with signs and/or symptoms suggestive of thyroid dysfunction should have laboratory monitoring of thyroid function performed and be treated as per standard medical practice.
Treatment-emergent acquired hypothyroidism was noted in eight GIST patients (4%) on SUTENT versus one (1%) on placebo. Hypothyroidism was reported as an adverse reaction in sixty-one patients (16%) on SUTENT in the treatment-naïve RCC study and in three patients (1%) in the IFN-α arm. Hypothyroidism was reported as an adverse reaction in 6/83 patients (7%) on SUTENT in the Phase 3 pNET study and in 1/82 patients (1%) in the placebo arm.
Cases of hyperthyroidism, some followed by hypothyroidism, have been reported in clinical trials and through post-marketing experience.
5.10 Wound Healing
Cases of impaired wound healing have been reported during SUTENT therapy. Temporary interruption of SUTENT therapy is recommended for precautionary reasons in patients undergoing major surgical procedures. There is limited clinical experience regarding the timing of reinitiation of therapy following major surgical intervention. Therefore, the decision to resume SUTENT therapy following a major surgical intervention should be based upon clinical judgment of recovery from surgery.
5.11 Adrenal Function
Physicians prescribing SUTENT are advised to monitor for adrenal insufficiency in patients who experience stress such as surgery, trauma or severe infection.
Adrenal toxicity was noted in non-clinical repeat dose studies of 14 days to 9 months in rats and monkeys at plasma exposures as low as 0.7 times the AUC observed in clinical studies. Histological changes of the adrenal gland were characterized as hemorrhage, necrosis, congestion, hypertrophy and inflammation. In clinical studies, CT/MRI obtained in 336 patients after exposure to one or more cycles of SUTENT demonstrated no evidence of adrenal hemorrhage or necrosis. ACTH stimulation testing was performed in approximately 400 patients across multiple clinical trials of SUTENT. Among patients with normal baseline ACTH stimulation testing, one patient developed consistently abnormal test results during treatment that are unexplained and may be related to treatment with SUTENT. Eleven additional patients with normal baseline testing had abnormalities in the final test performed, with peak cortisol levels of 12–16.4 mcg/dL (normal >18 mcg/dL) following stimulation. None of these patients were reported to have clinical evidence of adrenal insufficiency.
5.12 Laboratory Tests
CBCs with platelet count and serum chemistries including phosphate should be performed at the beginning of each treatment cycle for patients receiving treatment with SUTENT.
6 ADVERSE REACTIONS
The data described below reflect exposure to SUTENT in 660 patients who participated in the double-blind treatment phase of a placebo-controlled trial (n=202) for the treatment of GIST [see Clinical Studies (14.1)], an active-controlled trial (n=375) for the treatment of RCC [see Clinical Studies (14.2)] or a placebo-controlled trial (n=83) for the treatment of pNET [see Clinical Studies (14.3)]. The GIST and RCC patients received a starting oral dose of 50 mg daily on Schedule 4/2 in repeated cycles, and the pNET patients received a starting oral dose of 37.5 mg daily without scheduled off-treatment periods.
The most common adverse reactions (≥20%) in patients with GIST, RCC or pNET are fatigue, asthenia, fever, diarrhea, nausea, mucositis/stomatitis, vomiting, dyspepsia, abdominal pain, constipation, hypertension, peripheral edema, rash, hand-foot syndrome, skin discoloration, dry skin, hair color changes, altered taste, headache, back pain, arthralgia, extremity pain, cough, dyspnea, anorexia, and bleeding. The potentially serious adverse reactions of hepatotoxicity, left ventricular dysfunction, QT interval prolongation, hemorrhage, hypertension, thyroid dysfunction, and adrenal function are discussed in Warnings and Precautions (5) . Other adverse reactions occurring in GIST, RCC and pNET studies are described below.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Side Effects in GIST Study A
Median duration of blinded study treatment was two cycles for patients on SUTENT (mean 3.0, range 1–9) and one cycle (mean 1.8, range 1–6) for patients on placebo at the time of the interim analysis. Dose reductions occurred in 23 patients (11%) on SUTENT and none on placebo. Dose interruptions occurred in 59 patients (29%) on SUTENT and 31 patients (30%) on placebo. The rates of treatment-emergent, non-fatal adverse reactions resulting in permanent discontinuation were 7% and 6% in the SUTENT and placebo groups, respectively.
Most treatment-emergent adverse reactions in both study arms were Grade 1 or 2 in severity. Grade 3 or 4 treatment-emergent adverse reactions were reported in 56% versus 51% of patients on SUTENT versus placebo, respectively, in the double-blind treatment phase of the trial. Table 1 compares the incidence of common (≥10%) treatment-emergent adverse reactions for patients receiving SUTENT and reported more commonly in patients receiving SUTENT than in patients receiving placebo.
| Adverse Reaction, n (%) |
GIST | |||
|---|---|---|---|---|
| SUTENT (n=202) | Placebo (n=102) | |||
| All Grades | Grade 3/4 | All Grades | Grade 3/4 | |
| Any | 114 (56) | 52 (51) | ||
| Gastrointestinal | ||||
| Diarrhea | 81 (40) | 9 (4) | 27 (27) | 0 (0) |
| Mucositis/stomatitis | 58 (29) | 2 (1) | 18 (18) | 2 (2) |
| Constipation | 41 (20) | 0 (0) | 14 (14) | 2 (2) |
| Cardiac | ||||
| Hypertension | 31 (15) | 9 (4) | 11 (11) | 0 (0) |
| Dermatology | ||||
| Skin discoloration | 61 (30) | 0 (0) | 23 (23) | 0 (0) |
| Rash | 28 (14) | 2 (1) | 9 (9) | 0 (0) |
| Hand-foot syndrome | 28 (14) | 9 (4) | 10 (10) | 3 (3) |
| Neurology | ||||
| Altered taste | 42 (21) | 0 (0) | 12 (12) | 0 (0) |
| Musculoskeletal | ||||
| Myalgia/limb pain | 28 (14) | 1 (1) | 9 (9) | 1 (1) |
| Metabolism/Nutrition | ||||
Anorexia |
67 (33) | 1 (1) | 30 (29) | 5 (5) |
| Asthenia | 45 (22) | 10 (5) | 11 (11) | 3 (3) |
In the double-blind treatment phase of GIST Study A, oral pain other than mucositis/stomatitis occurred in 12 patients (6%) on SUTENT versus 3 (3%) on placebo. Hair color changes occurred in 15 patients (7%) on SUTENT versus 4 (4%) on placebo. Alopecia was observed in 10 patients (5%) on SUTENT versus 2 (2%) on placebo.
Table 2 provides common (≥10%) treatment-emergent laboratory abnormalities.
| Laboratory Parameter, n (%) | GIST | |||
|---|---|---|---|---|
| SUTENT (n=202) | Placebo (n=102) | |||
All Grades |
Grade 3/4  |
All Grades |
Grade 3/4  |
|
| LVEF=Left ventricular ejection fraction | ||||
| Any | 68 (34) | 22 (22) | ||
| Gastrointestinal | ||||
| AST / ALT | 78 (39) | 3 (2) | 23 (23) | 1 (1) |
| Lipase | 50 (25) | 20 (10) | 17 (17) | 7 (7) |
| Alkaline phosphatase | 48 (24) | 7 (4) | 21 (21) | 4 (4) |
| Amylase | 35 (17) | 10 (5) | 12 (12) | 3 (3) |
| Total bilirubin | 32 (16) | 2 (1) | 8 (8) | 0 (0) |
| Indirect bilirubin | 20 (10) | 0 (0) | 4 (4) | 0 (0) |
| Cardiac | ||||
| Decreased LVEF | 22 (11) | 2 (1) | 3 (3) | 0 (0) |
| Renal/Metabolic | ||||
| Creatinine | 25 (12) | 1 (1) | 7 (7) | 0 (0) |
| Potassium decreased | 24 (12) | 1 (1) | 4 (4) | 0 (0) |
| Sodium increased | 20 (10) | 0 (0) | 4 (4) | 1 (1) |
| Hematology | ||||
| Neutrophils | 107 (53) | 20 (10) | 4 (4) | 0 (0) |
| Lymphocytes | 76 (38) | 0 (0) | 16 (16) | 0 (0) |
| Platelets | 76 (38) | 10 (5) | 4 (4) | 0 (0) |
| Hemoglobin | 52 (26) | 6 (3) | 22 (22) | 2 (2) |
After an interim analysis, the study was unblinded, and patients on the placebo arm were given the opportunity to receive open-label SUTENT treatment [see Clinical Studies (14.1)]. For 241 patients randomized to the SUTENT arm, including 139 who received SUTENT in both the double-blind and open-label treatment phases, the median duration of SUTENT treatment was 6 cycles (mean 8.5, range 1 – 44). For the 255 patients who ultimately received open-label SUTENT treatment, median duration of study treatment was 6 cycles (mean 7.8, range 1 – 37) from the time of the unblinding. A total of 118 patients (46%) required dosing interruptions, and a total of 72 patients (28%) required dose reductions. The incidence of treatment-emergent adverse reactions resulting in permanent discontinuation was 20%. The most common Grade 3 or 4 treatment-related adverse reactions experienced by patients receiving SUTENT in the open-label treatment phase were fatigue (10%), hypertension (8%), asthenia (5%), diarrhea (5%), hand-foot syndrome (5%), nausea (4%), abdominal pain (3%), anorexia (3%), mucositis (2%), vomiting (2%), and hypothyroidism (2%).
6.2 Side Effects in the Treatment-Naïve RCC Study
The as-treated patient population for the treatment-naive RCC study included 735 patients, 375 randomized to SUTENT and 360 randomized to IFN-α. The median duration of treatment was 11.1 months (range: 0.4 – 46.1) for SUTENT treatment and 4.1 months (range: 0.1 – 45.6) for IFN-α treatment. Dose interruptions occurred in 202 patients (54%) on SUTENT and 141 patients (39%) on IFN-α. Dose reductions occurred in 194 patients (52%) on SUTENT and 98 patients (27%) on IFN-α. Discontinuation rates due to adverse reactions were 20% for SUTENT and 24% for IFN-α. Most treatment-emergent adverse reactions in both study arms were Grade 1 or 2 in severity. Grade 3 or 4 treatment-emergent adverse reactions were reported in 77% versus 55% of patients on SUTENT versus IFN-α, respectively.
Table 3 compares the incidence of common (≥10%) treatment-emergent adverse reactions for patients receiving SUTENT versus IFN-α.
| Adverse Reaction, n (%) |
Treatment-Naïve RCC | |||
|---|---|---|---|---|
| SUTENT (n=375) | IFN-α (n=360) | |||
| All Grades | Grade 3/4 |
All Grades | Grade 3/4 |
|
| Any | 372 (99) | 290 (77) | 355 (99) | 197 (55) |
| Constitutional | ||||
| Fatigue | 233 (62) | 55 (15) | 202 (56) | 54 (15) |
| Asthenia | 96 (26) | 42 (11) | 81 (22) | 21 (6) |
| Fever | 84 (22) | 3 (1) | 134 (37) | 1 (<1) |
| Weight decreased | 60 (16) | 1 (<1) | 60 (17) | 3 (1) |
| Chills | 53 (14) | 3 (1) | 111 (31) | 0 (0) |
| Chest Pain | 50 (13) | 7 (2) | 24 (7) | 3 (1) |
| Influenza like illness | 18 (5) | 0 (0) | 54 (15) | 1 (<1) |
| Gastrointestinal | ||||
| Diarrhea | 246 (66) | 37 (10) | 76 (21) | 1 (<1) |
| Nausea | 216 (58) | 21 (6) | 147 (41) | 6 (2) |
| Mucositis/stomatitis | 178 (47) | 13 (3) | 19 (5) | 2 (<1) |
| Vomiting | 148 (39) | 19 (5) | 62 (17) | 4 (1) |
| Dyspepsia | 128 (34) | 8 (2) | 16 (4) | 0 (0) |
Abdominal pain |
113 (30) | 20 (5) | 42 (12) | 5 (1) |
| Constipation | 85 (23) | 4 (1) | 49 (14) | 1 (<1) |
| Dry mouth | 50 (13) | 0 (0) | 27 (7) | 1 (<1) |
| GERD/reflux esophagitis | 47 (12) | 1 (<1) | 3 (1) | 0(0) |
| Flatulence | 52 (14) | 0 (0) | 8 (2) | 0 (0) |
| Oral pain | 54 (14) | 2 (<1) | 2 (1) | 0 (0) |
| Glossodynia | 40 (11) | 0 (0) | 2 (1) | 0 (0) |
| Hemorrhoids | 38 (10) | 0 (0) | 6 (2) | 0 (0) |
| Cardiac | ||||
| Hypertension | 127 (34) | 50 (13) | 13 (4) | 1 (<1) |
| Edema, peripheral | 91 (24) | 7 (2) | 17 (5) | 2 (1) |
| Ejection fraction decreased | 61 (16) | 10 (3) | 19 (5) | 6 (2) |
| Dermatology | ||||
| Rash | 109 (29) | 6 (2) | 39 (11) | 1 (<1) |
| Hand-foot syndrome | 108 (29) | 32 (8) | 3 (1) | 0 (0) |
| Skin discoloration/ yellow skin | 94 (25) | 1 (<1) | 0 (0) | 0 (0) |
| Dry skin | 85 (23) | 1 (<1) | 26 (7) | 0 (0) |
| Hair color changes | 75 (20) | 0 (0) | 1 (<1) | 0 (0) |
| Alopecia | 51 (14) | 0 (0) | 34 (9) | 0 (0) |
| Erythema | 46 (12) | 2 (<1) | 5 (1) | 0 (0) |
| Pruritus | 44 (12) | 1 (<1) | 24 (7) | 1 (<1) |
| Neurology | ||||
Altered taste |
178 (47) | 1 (<1) | 54 (15) | 0 (0) |
| Headache | 86 (23) | 4 (1) | 69 (19) | 0 (0) |
| Dizziness | 43 (11) | 2 (<1) | 50 (14) | 2 (1) |
| Musculoskeletal | ||||
| Back pain | 105 (28) | 19 (5) | 52 (14) | 7 (2) |
| Arthralgia | 111 (30) | 10 (3) | 69 (19) | 4 (1) |
| Pain in extremity/ limb discomfort | 150 (40) | 19 (5) | 107 (30) | 7 (2) |
| Endocrine | ||||
| Hypothyroidism | 61 (16) | 6 (2) | 3 (1) | 0 (0) |
| Respiratory | ||||
| Cough | 100 (27) | 3 (1) | 51 (14) | 1 (<1) |
| Dyspnea | 99 (26) | 24 (6) | 71 (20) | 15 (4) |
| Nasopharyngitis | 54 (14) | 0 (0) | 8 (2) | 0 (0) |
| Oropharyngeal Pain | 51 (14) | 2 (<1) | 9 (2) | 0 (0) |
| Upper respiratory tract infection | 43 (11) | 2 (<1) | 9 (2) | 0 (0) |
| Metabolism/Nutrition | ||||
Anorexia |
182 (48) | 11 (3) | 153 (42) | 7 (2) |
| Hemorrhage/Bleeding | ||||
| Bleeding, all sites | 140 (37) | 16 (4) |
35 (10) | 3 (1) |
| Psychiatric | ||||
| Insomnia | 57 (15) | 3 (<1) | 37 (10) | 0 (0) |
Depression |
40 (11) | 0 (0) | 51 (14) | 5 (1) |
Treatment-emergent Grade 3/4 laboratory abnormalities are presented in Table 4.
| Laboratory Parameter, n (%) | Treatment-Naïve RCC | |||
|---|---|---|---|---|
| SUTENT (n=375) | IFN-α (n=360) | |||
All Grades |
Grade 3/4  |
All Grades |
Grade 3/4  |
|
| Gastrointestinal | ||||
| AST | 211 (56) | 6 (2) | 136 (38) | 8 (2) |
| ALT | 192 (51) | 10 (3) | 144 (40) | 9 (2) |
| Lipase | 211 (56) | 69 (18) | 165 (46) | 29 (8) |
| Alkaline phosphatase | 171 (46) | 7 (2) | 132 (37) | 6 (2) |
| Amylase | 130 (35) | 22 (6) | 114 (32) | 12 (3) |
| Total bilirubin | 75 (20) | 3 (1) | 8 (2) | 0 (0) |
| Indirect bilirubin | 49 (13) | 4 (1) | 3 (1) | 0 (0) |
| Renal/Metabolic | ||||
| Creatinine | 262 (70) | 2 (<1) | 183 (51) | 1 (<1) |
| Creatine kinase | 183 (49) | 9 (2) | 40 (11) | 4 (1) |
| Uric acid | 173 (46) | 54 (14) | 119 (33) | 29 (8) |
| Calcium decreased | 156 (42) | 4 (1) | 145 (40) | 4 (1) |
| Phosphorus | 116 (31) | 22 (6) | 87 (24) | 23 (6) |
| Albumin | 106 (28) | 4 (1) | 72 (20) | 0 (0) |
| Glucose increased | 86 (23) | 21 (6) | 55 (15) | 22 (6) |
| Sodium decreased | 75 (20) | 31 (8) | 55 (15) | 13 (4) |
| Glucose decreased | 65 (17) | 0 (0) | 43 (12) | 1 (<1) |
| Potassium increased | 61 (16) | 13 (3) | 61 (17) | 15 (4) |
| Calcium increased | 50 (13) | 2 (<1) | 35 (10) | 5 (1) |
| Potassium decreased | 49 (13) | 3 (1) | 7 (2) | 1 (<1) |
| Sodium increased | 48 (13) | 0 (0) | 38 (10) | 0 (0) |
| Hematology | ||||
| Neutrophils | 289 (77) | 65 (17) | 178 (49) | 31 (9) |
| Hemoglobin | 298 (79) | 29 (8) | 250 (69) | 18 (5) |
| Platelets | 255 (68) | 35 (9) | 85 (24) | 2 (1) |
| Lymphocytes | 256 (68) | 66 (18) | 245 (68) | 93 (26) |
| Leukocytes | 293 (78) | 29 (8) | 202 (56) | 8 (2) |
6.3 Side Effects in the Phase 3 pNET Study
The median number of days on treatment was 139 days (range 13–532 days) for patients on SUTENT and 113 days (range 1–614 days) for patients on placebo. Nineteen patients (23%) on SUTENT and 4 patients (5%) on placebo were on study for >1 year. Dose interruptions occurred in 25 patients (30%) on SUTENT and 10 patients (12%) on placebo. Dose reductions occurred in 26 patients (31%) on SUTENT and 9 patients (11%) on placebo. Discontinuation rates due to adverse reactions were 22% for SUTENT and 17% for placebo.
Most treatment-emergent adverse reactions in both study arms were Grade 1 or 2 in severity. Grade 3 or 4 treatment-emergent adverse reactions were reported in 54% versus 50% of patients on SUTENT versus placebo, respectively. Table 5 compares the incidence of common (≥10%) treatment-emergent adverse reactions for patients receiving SUTENT and reported more commonly in patients receiving SUTENT than in patients receiving placebo.
| Adverse Reaction, n (%) |
pNET | |||
|---|---|---|---|---|
| SUTENT (n=83) | Placebo (n=82) | |||
| All Grades | Grade 3/4 |
All Grades | Grade 3/4 | |
| Any | 82 (99) | 45 (54) | 78 (95) | 41 (50) |
| Constitutional | ||||
| Asthenia | 28 (34) | 4 (5) | 22 (27) | 3 (4) |
| Fatigue | 27 (33) | 4 (5) | 22 (27) | 7 (9) |
| Weight decreased | 13 (16) | 1(1) | 9 (11) | 0 (0) |
| Gastrointestinal | ||||
| Diarrhea | 49 (59) | 4 (5) | 32 (39) | 2 (2) |
Stomatitis/oral Syndromes |
40 (48) | 5 (6) | 15 (18) | 0 (0) |
| Nausea | 37 (45) | 1 (1) | 24 (29) | 1 (1) |
Abdominal pain |
32 (39) | 4 (5) | 28 (34) | 8 (10) |
| Vomiting | 28 (34) | 0 (0) | 25 (31) | 2 (2) |
| Dyspepsia | 12 (15) | 0 (0) | 5 (6) | 0 (0) |
| Cardiac | ||||
| Hypertension | 22 (27) | 8 (10) | 4 (5) | 1 (1) |
| Dermatology | ||||
| Hair color changes | 24 (29) | 1 (1) | 1 (1) | 0 (0) |
| Hand-foot syndrome | 19 (23) | 5 (6) | 2 (2) | 0 (0) |
| Rash | 15 (18) | 0 (0) | 4 (5) | 0 (0) |
| Dry skin | 12 (15) | 0 (0) | 9 (11) | 0 (0) |
| Neurology | ||||
| Dysgeusia | 17 (21) | 0 (0) | 4 (5) | 0 (0) |
| Headache | 15 (18) | 0 (0) | 11 (13) | 1 (1) |
| Musculoskeletal | ||||
| Arthralgia | 12 (15) | 0 (0) | 5 (6) | 0 (0) |
| Psychiatric | ||||
| Insomnia | 15 (18) | 0 (0) | 10 (12) | 0 (0) |
| Hemorrhage/Bleeding | ||||
Bleeding events |
18 (22) | 0 (0) | 8 (10) | 3 (4) |
| Epistaxis | 17 (21) | 1 (1) | 4 (5) | 0 (0) |
Table 6 provides common (≥10%) treatment-emergent laboratory abnormalities.
| Laboratory Parameter, n (%) | pNET | |||||
|---|---|---|---|---|---|---|
| SUTENT | Placebo | |||||
| N | All Grades |
Grade 3/4  |
N | All Grades |
Grade 3/4  |
|
| Gastrointestinal | ||||||
| AST increased | 82 | 59 (72) | 4 (5) | 80 | 56 (70) | 2 (3) |
| ALT increased | 82 | 50 (61) | 3 (4) | 80 | 44 (55) | 2 (3) |
| Alkaline phosphatase increased | 82 | 52 (63) | 8 (10) | 80 | 56 (70) | 9 (11) |
| Total bilirubin increased | 82 | 30 (37) | 1 (1) | 80 | 22 (28) | 3 (4) |
| Amylase increased | 74 | 15 (20) | 3 (4) | 74 | 7 (10) | 1 (1) |
| Lipase increased | 75 | 13 (17) | 4 (5) | 72 | 8 (11) | 3 (4) |
| Renal/Metabolic | ||||||
| Glucose increased | 82 | 58 (71) | 10 (12) | 80 | 62 (78) | 14 (18) |
| Albumin decreased | 81 | 33 (41) | 1 (1) | 79 | 29 (37) | 1 (1) |
| Phosphorus decreased | 81 | 29 (36) | 6 (7) | 77 | 17 (22) | 4 (5) |
| Calcium decreased | 82 | 28 (34) | 0 (0) | 80 | 15 (19) | 0 (0) |
| Sodium decreased | 82 | 24 (29) | 2 (2) | 80 | 27 (34) | 2 (3) |
| Creatinine increased | 82 | 22 (27) | 4 (5) | 80 | 22 (28) | 4 (5) |
| Glucose decreased | 82 | 18 (22) | 2 (2) | 80 | 12 (15) | 3 (4) |
| Potassium decreased | 82 | 17 (21) | 3 (4) | 80 | 11 (14) | 0 (0) |
| Magnesium decreased | 52 | 10 (19) | 0 (0) | 39 | 4 (10) | 0 (0) |
| Potassium increased | 82 | 15 (18) | 1 (1) | 80 | 9 (11) | 1 (1) |
| Hematology | ||||||
| Neutrophils decreased | 82 | 58 (71) | 13 (16) | 80 | 13 (16) | 0 (0) |
| Hemoglobin decreased | 82 | 53 (65) | 0 (0) | 80 | 44 (55) | 1 (1) |
| Platelets decreased | 82 | 49 (60) | 4 (5) | 80 | 12 (15) | 0 (0) |
| Lymphocytes decreased | 82 | 46 (56) | 6 (7) | 80 | 28 (35) | 3 (4) |
6.4 Venous Thromboembolic Events
Seven patients (3%) on SUTENT and none on placebo in the double-blind treatment phase of GIST Study A experienced venous thromboembolic events; five of the seven were Grade 3 deep venous thrombosis (DVT), and two were Grade 1 or 2. Four of these seven GIST patients discontinued treatment following first observation of DVT.
Thirteen (3%) patients receiving SUTENT for treatment-naïve RCC had venous thromboembolic events reported. Seven (2%) of these patients had pulmonary embolism, one was Grade 2 and six were Grade 4, and six (2%) patients had DVT, including three Grade 3. One patient was permanently withdrawn from SUTENT due to pulmonary embolism; dose interruption occurred in two patients with pulmonary embolism and one with DVT. In treatment-naïve RCC patients receiving IFN-α, six (2%) venous thromboembolic events occurred; one patient (<1%) experienced a Grade 3 DVT and five patients (1%) had pulmonary embolism, all Grade 4. One patient (1%) receiving SUTENT for pNET had a venous thromboembolic event reported compared to 5 patients (6%) receiving placebo. The SUTENT patient had Grade 2 thrombosis. Two placebo patients had DVT, one was Grade 3, two placebo patients had pulmonary embolism, one was Grade 3 and one was Grade 4, and one placebo patient had Grade 3 jugular thrombosis.
6.5 Reversible Posterior Leukoencephalopathy Syndrome
There have been reports (<1%), some fatal, of subjects presenting with seizures and radiological evidence of reversible posterior leukoencephalopathy syndrome (RPLS). Patients with seizures and signs/symptoms consistent with RPLS, such as hypertension, headache, decreased alertness, altered mental functioning, and visual loss, including cortical blindness should be controlled with medical management including control of hypertension. Temporary suspension of SUTENT is recommended; following resolution, treatment may be resumed at the discretion of the treating physician.
6.6 Pancreatic and Hepatic Function
If symptoms of pancreatitis or hepatic failure are present, patients should have SUTENT discontinued. Pancreatitis was observed in 5 (1%) patients receiving SUTENT for treatment-naïve RCC compared to 1 (<1%) patient receiving IFN-α. Pancreatitis was observed in 1 (1%) patient receiving SUTENT for pNET and 1 (1%) patient receiving placebo. Hepatotoxicity was observed in patients receiving SUTENT [See Boxed Warning and Warnings and Precautions (5.1)].
6.7 Post-marketing Experience
The following adverse reactions have been identified during post-approval use of SUTENT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: thrombotic microangiopathy; hemorrhage associated with thrombocytopenia
Gastrointestinal disorders: esophagitis
Hepatobiliary disorders: cholecystitis, particularly acalculous cholecystitis.
Immune system disorders: hypersensitivity reactions, including angioedema.
Infections and infestations: serious infection (with or without neutropenia)

Musculoskeletal and connective tissue disorders: fistula formation, sometimes associated with tumor necrosis and/or regression

Renal and urinary disorders: renal impairment and/or failure
Respiratory disorders: pulmonary embolism
Skin and subcutaneous tissue disorders: pyoderma gangrenosum, including positive dechallenges; erythema multiforme and Stevens-Johnson syndrome.
Vascular disorders: arterial thromboembolic events
7 DRUG INTERACTIONS
7.1 CYP3A4 Inhibitors
Strong CYP3A4 inhibitors such as ketoconazole may increase sunitinib plasma concentrations. Selection of an alternate concomitant medication with no or minimal enzyme inhibition potential is recommended. Concurrent administration of SUTENT with the strong CYP3A4 inhibitor, ketoconazole, resulted in 49% and 51% increases in the combined (sunitinib + primary active metabolite) Cmax and AUC0– ∞ values, respectively, after a single dose of SUTENT in healthy volunteers. Co-administration of SUTENT with strong inhibitors of the CYP3A4 family (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, voriconazole) may increase sunitinib concentrations. Grapefruit may also increase plasma concentrations of sunitinib. A dose reduction for SUTENT should be considered when it must be co-administered with strong CYP3A4 inhibitors [see Dosage and Administration (2.2)].
7.2 CYP3A4 Inducers
CYP3A4 inducers such as rifampin may decrease sunitinib plasma concentrations. Selection of an alternate concomitant medication with no or minimal enzyme induction potential is recommended. Concurrent administration of SUTENT with the strong CYP3A4 inducer, rifampin, resulted in a 23% and 46% reduction in the combined (sunitinib + primary active metabolite) Cmax and AUC0– ∞ values, respectively, after a single dose of SUTENT in healthy volunteers. Co-administration of SUTENT with inducers of the CYP3A4 family (e.g., dexamethasone, phenytoin, carbamazepine, rifampin, rifabutin, rifapentin, phenobarbital, St. John's Wort) may decrease sunitinib concentrations. St. John's Wort may decrease sunitinib plasma concentrations unpredictably. Patients receiving SUTENT should not take St. John's Wort concomitantly. A dose increase for SUTENT should be considered when it must be co-administered with CYP3A4 inducers [see Dosage and Administration (2.2)].
7.3 In Vitro Studies of CYP Inhibition and Induction
In vitro studies indicated that sunitinib does not induce or inhibit major CYP enzymes. The in vitro studies in human liver microsomes and hepatocytes of the activity of CYP isoforms CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4/5, and CYP4A9/11 indicated that sunitinib and its primary active metabolite are unlikely to have any clinically relevant drug-drug interactions with drugs that may be metabolized by these enzymes.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D [see Warnings and Precautions (5.2)].
SUTENT can cause fetal harm when administered to a pregnant woman. As angiogenesis is a critical component of embryonic and fetal development, inhibition of angiogenesis following administration of SUTENT should be expected to result in adverse effects on pregnancy. In animal reproductive studies in rats and rabbits, sunitinib was teratogenic, embryotoxic, and fetotoxic. There are no adequate and well-controlled studies of SUTENT in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus. Women of childbearing potential should be advised to avoid becoming pregnant while receiving treatment with SUTENT.
Sunitinib was evaluated in pregnant rats (0.3, 1.5, 3.0, 5.0 mg/kg/day) and rabbits (0.5, 1, 5, 20 mg/kg/day) for effects on the embryo. Significant increases in the incidence of embryolethality and structural abnormalities were observed in rats at the dose of 5 mg/kg/day (approximately 5.5 times the systemic exposure [combined AUC of sunitinib + primary active metabolite] in patients administered the recommended daily doses [RDD]). Significantly increased embryolethality was observed in rabbits at 5 mg/kg/day while developmental effects were observed at ≥1 mg/kg/day (approximately 0.3 times the AUC in patients administered the RDD of 50 mg/day). Developmental effects consisted of fetal skeletal malformations of the ribs and vertebrae in rats. In rabbits, cleft lip was observed at 1 mg/kg/day and cleft lip and cleft palate were observed at 5 mg/kg/day (approximately 2.7 times the AUC in patients administered the RDD). Neither fetal loss nor malformations were observed in rats dosed at ≤3 mg/kg/day (approximately 2.3 times the AUC in patients administered the RDD).
Sunitinib (0.3, 1.0, 3.0 mg/kg/day) was evaluated in a pre- and postnatal development study in pregnant rats. Maternal body weight gains were reduced during gestation and lactation at doses ≥1 mg/kg/day but no maternal reproductive toxicity was observed at doses up to 3 mg/kg/day (approximately 2.3 times the AUC in patients administered the RDD). At the high dose of 3 mg/kg/day, reduced body weights were observed at birth and persisted for offspring of both sexes during the pre-weaning period and in males during post-weaning period. No other developmental toxicity was observed at doses up to 3 mg/kg/day (approximately 2.3 times the AUC in patients administered the RDD).
8.3 Nursing Mothers
Sunitinib and its metabolites are excreted in rat milk. In lactating female rats administered 15 mg/kg, sunitinib and its metabolites were extensively excreted in milk at concentrations up to 12-fold higher than in plasma. It is not known whether this drug or its primary active metabolite are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from SUTENT, a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and efficacy of SUTENT in pediatric patients have not been established.
Physeal dysplasia was observed in cynomolgus monkeys with open growth plates treated for ≥3 months (3 month dosing 2, 6, 12 mg/kg/day; 8 cycles of dosing 0.3, 1.5, 6.0 mg/kg/day) with sunitinib at doses that were >0.4 times the RDD based on systemic exposure (AUC). In developing rats treated continuously for 3 months (1.5, 5.0 and 15.0 mg/kg) or 5 cycles (0.3, 1.5, and 6.0 mg/kg/day), bone abnormalities consisted of thickening of the epiphyseal cartilage of the femur and an increase of fracture of the tibia at doses ≥5 mg/kg (approximately 10 times the RDD based on AUC). Additionally, caries of the teeth were observed in rats at >5 mg/kg. The incidence and severity of physeal dysplasia were dose-related and were reversible upon cessation of treatment; however, findings in the teeth were not. A no effect level was not observed in monkeys treated continuously for 3 months, but was 1.5 mg/kg/day when treated intermittently for 8 cycles. In rats the no effect level in bones was ≤2 mg/kg/day.
8.5 Geriatric Use
Of 825 GIST and RCC patients who received SUTENT on clinical studies, 277 (34%) were 65 and over. In the Phase 3 pNET study, 22 (27%) patients who received SUTENT were 65 and over. No overall differences in safety or effectiveness were observed between younger and older patients.
8.6 Hepatic Impairment
No dose adjustment to the starting dose is required when administering SUTENT to patients with Child-Pugh Class A or B hepatic impairment. Sunitinib and its primary metabolite are primarily metabolized by the liver. Systemic exposures after a single dose of SUTENT were similar in subjects with mild or moderate (Child-Pugh Class A and B) hepatic impairment compared to subjects with normal hepatic function. SUTENT was not studied in subjects with severe (Child-Pugh Class C) hepatic impairment. Studies in cancer patients have excluded patients with ALT or AST >2.5 × ULN or, if due to liver metastases, >5.0 × ULN.
8.7 Renal Impairment
No adjustment to the starting dose is required when administering SUTENT to patients with mild, moderate, and severe renal impairment. Subsequent dose modifications should be based on safety and tolerability [see Dose Modification (2.3)]. In patients with end-stage renal disease (ESRD) on hemodialysis, no adjustment to the starting dose is required. However, compared to subjects with normal renal function, the sunitinib exposure is 47% lower in subjects with ESRD on hemodialysis. Therefore, the subsequent doses may be increased gradually up to 2 fold based on safety and tolerability.
10 OVERDOSAGE
Treatment of overdose with SUTENT should consist of general supportive measures. There is no specific antidote for overdosage with SUTENT. If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage. A few cases of accidental overdose have been reported; these cases were associated with adverse reactions consistent with the known safety profile of SUTENT, or without adverse reactions. A case of intentional overdose involving the ingestion of 1,500 mg of SUTENT in an attempted suicide was reported without adverse reaction. In non-clinical studies mortality was observed following as few as 5 daily doses of 500 mg/kg (3000 mg/m2) in rats. At this dose, signs of toxicity included impaired muscle coordination, head shakes, hypoactivity, ocular discharge, piloerection and gastrointestinal distress. Mortality and similar signs of toxicity were observed at lower doses when administered for longer durations.
11 DESCRIPTION
SUTENT, an oral multi-kinase inhibitor, is the malate salt of sunitinib. Sunitinib malate is described chemically as Butanedioic acid, hydroxy-, (2S)-, compound with N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidine)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (1:1). The molecular formula is C22H27FN4O2 • C4H6O5 and the molecular weight is 532.6 Daltons.
The chemical structure of sunitinib malate is:
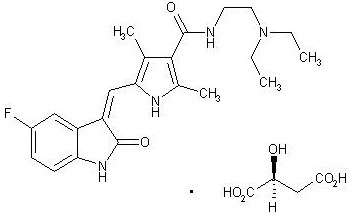
Sunitinib malate is a yellow to orange powder with a pKa of 8.95. The solubility of sunitinib malate in aqueous media over the range pH 1.2 to pH 6.8 is in excess of 25 mg/mL. The log of the distribution coefficient (octanol/water) at pH 7 is 5.2.
SUTENT (sunitinib malate) capsules are supplied as printed hard shell capsules containing sunitinib malate equivalent to 12.5 mg, 25 mg or 50 mg of sunitinib together with mannitol, croscarmellose sodium, povidone (K-25) and magnesium stearate as inactive ingredients.
The orange gelatin capsule shells contain titanium dioxide, and red iron oxide. The caramel gelatin capsule shells contain titanium dioxide, red iron oxide, yellow iron oxide and black iron oxide. The white printing ink contains shellac, propylene glycol, sodium hydroxide, povidone and titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Sunitinib is a small molecule that inhibits multiple receptor tyrosine kinases (RTKs), some of which are implicated in tumor growth, pathologic angiogenesis, and metastatic progression of cancer. Sunitinib was evaluated for its inhibitory activity against a variety of kinases (>80 kinases) and was identified as an inhibitor of platelet-derived growth factor receptors (PDGFRα and PDGFRβ), vascular endothelial growth factor receptors (VEGFR1, VEGFR2 and VEGFR3), stem cell factor receptor (KIT), Fms-like tyrosine kinase-3 (FLT3), colony stimulating factor receptor Type 1 (CSF-1R), and the glial cell-line derived neurotrophic factor receptor (RET). Sunitinib inhibition of the activity of these RTKs has been demonstrated in biochemical and cellular assays, and inhibition of function has been demonstrated in cell proliferation assays. The primary metabolite exhibits similar potency compared to sunitinib in biochemical and cellular assays.
Sunitinib inhibited the phosphorylation of multiple RTKs (PDGFRβ, VEGFR2, KIT) in tumor xenografts expressing RTK targets in vivo and demonstrated inhibition of tumor growth or tumor regression and/or inhibited metastases in some experimental models of cancer. Sunitinib demonstrated the ability to inhibit growth of tumor cells expressing dysregulated target RTKs (PDGFR, RET, or KIT) in vitro and to inhibit PDGFRβ- and VEGFR2-dependent tumor angiogenesis in vivo.
12.3 Pharmacokinetics
The pharmacokinetics of sunitinib and sunitinib malate have been evaluated in 135 healthy volunteers and in 266 patients with solid tumors.
Maximum plasma concentrations (Cmax) of sunitinib are generally observed between 6 and 12 hours (Tmax) following oral administration. Food has no effect on the bioavailability of sunitinib. SUTENT may be taken with or without food.
Binding of sunitinib and its primary active metabolite to human plasma protein in vitro was 95% and 90%, respectively, with no concentration dependence in the range of 100 – 4000 ng/mL. The apparent volume of distribution (Vd/F) for sunitinib was 2230 L. In the dosing range of 25 – 100 mg, the area under the plasma concentration-time curve (AUC) and Cmax increase proportionately with dose.
Sunitinib is metabolized primarily by the cytochrome P450 enzyme, CYP3A4, to produce its primary active metabolite, which is further metabolized by CYP3A4. The primary active metabolite comprises 23 to 37% of the total exposure. Elimination is primarily via feces. In a human mass balance study of [14C]sunitinib, 61% of the dose was eliminated in feces, with renal elimination accounting for 16% of the administered dose. Sunitinib and its primary active metabolite were the major drug-related compounds identified in plasma, urine, and feces, representing 91.5%, 86.4% and 73.8% of radioactivity in pooled samples, respectively. Minor metabolites were identified in urine and feces but generally not found in plasma. Total oral clearance (CL/F) ranged from 34 to 62 L/hr with an inter-patient variability of 40%.
Following administration of a single oral dose in healthy volunteers, the terminal half-lives of sunitinib and its primary active metabolite are approximately 40 to 60 hours and 80 to 110 hours, respectively. With repeated daily administration, sunitinib accumulates 3- to 4-fold while the primary metabolite accumulates 7- to 10-fold. Steady-state concentrations of sunitinib and its primary active metabolite are achieved within 10 to 14 days. By Day 14, combined plasma concentrations of sunitinib and its active metabolite ranged from 62.9 – 101 ng/mL. No significant changes in the pharmacokinetics of sunitinib or the primary active metabolite were observed with repeated daily administration or with repeated cycles in the dosing regimens tested.
The pharmacokinetics were similar in healthy volunteers and in the solid tumor patient populations tested, including patients with GIST and RCC.
Pharmacokinetics in Special Populations
Population pharmacokinetic analyses of demographic data indicate that there are no clinically relevant effects of age, body weight, creatinine clearance, race, gender, or ECOG score on the pharmacokinetics of SUTENT or the primary active metabolite.
Pediatric Use: The pharmacokinetics of SUTENT have not been evaluated in pediatric patients.
Renal Insufficiency: Sunitinib systemic exposure after a single dose of SUTENT was similar in subjects with severe renal impairment (CLcr<30 mL/min) compared to subjects with normal renal function (CLcr>80 mL/min). Although sunitinib was not eliminated through hemodialysis, the sunitinib systemic exposure was 47% lower in subjects with ESRD on hemodialysis compared to subjects with normal renal function.
Hepatic Insufficiency: Systemic exposures after a single dose of SUTENT were similar in subjects with mild exocrine (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment compared to subjects with normal hepatic function.
12.4 Cardiac Electrophysiology
See Warnings and Precautions (5.4).
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of sunitinib has been evaluated in two species: rasH2 transgenic mice and Sprague-Dawley rats. There were similar positive findings in both species. In rasH2 transgenic mice gastroduodenal carcinomas and/or gastric mucosal hyperplasia, as well as an increased incidence of background hemangiosarcomas were observed at doses of ≥25 mg/kg/day following daily dose administration of sunitinib in studies of 1 or 6 months duration. No proliferative changes were observed in rasH2 transgenic mice at 8 mg/kg/day. Similarly, in a 2-year rat carcinogenicity study, administration of sunitinib in 28-day cycles followed by 7-day dose-free periods resulted in findings of duodenal carcinoma at doses as low as 1 mg/kg/day (approximately 0.9 times the AUC in patients given the RDD of 50 mg/day).At the high dose of 3 mg/kg/day (approximately 7.8 times the AUC in patients at the RDD of 50 mg/day) the incidence of duodenal tumors was increased and was accompanied by findings of gastric mucous cell hyperplasia and by an increased incidence of pheochromocytoma and hyperplasia of the adrenal. Sunitinib did not cause genetic damage when tested in in vitro assays (bacterial mutation [AMES Assay], human lymphocyte chromosome aberration) and an in vivo rat bone marrow micronucleus test.
Effects on the female reproductive system were identified in a 3-month repeat dose monkey study (2, 6, 12 mg/kg/day), where ovarian changes (decreased follicular development) were noted at 12 mg/kg/day (≥5.1 times the AUC in patients administered the RDD), while uterine changes (endometrial atrophy) were noted at ≥2 mg/kg/day (≥0.4 times the AUC in patients administered the RDD). With the addition of vaginal atrophy, the uterine and ovarian effects were reproduced at 6 mg/kg/day in the 9-month monkey study (0.3, 1.5 and 6 mg/kg/day administered daily for 28 days followed by a 14 day respite; the 6 mg/kg dose produced a mean AUC that was ≥0.8 times the AUC in patients administered the RDD). A no effect level was not identified in the 3 month study; 1.5 mg/kg/day represents a no effect level in monkeys administered sunitinib for 9 months.
Although fertility was not affected in rats, SUTENT may impair fertility in humans. In female rats, no fertility effects were observed at doses of ≤5.0 mg/kg/day [(0.5, 1.5, 5.0 mg/kg/day) administered for 21 days up to gestational day 7; the 5.0 mg/kg dose produced an AUC that was ≥5 times the AUC in patients administered the RDD], however significant embryolethality was observed at the 5.0 mg/kg dose. No reproductive effects were observed in male rats dosed (1, 3 or 10 mg/kg/day) for 58 days prior to mating with untreated females. Fertility, copulation, conception indices, and sperm evaluation (morphology, concentration, and motility) were unaffected by sunitinib at doses ≤10 mg/kg/day (the 10 mg/kg/day dose produced a mean AUC that was ≥25.8 times the AUC in patients administered the RDD).
14 CLINICAL STUDIES
14.1 Gastrointestinal Stromal Tumor
GIST Study A
Study A was a two-arm, international, randomized, double-blind, placebo-controlled trial of SUTENT in patients with GIST who had disease progression during prior imatinib mesylate (imatinib) treatment or who were intolerant of imatinib. The objective was to compare Time-to-Tumor Progression (TTP) in patients receiving SUTENT plus best supportive care versus patients receiving placebo plus best supportive care. Other objectives included Progression-Free Survival (PFS), Objective Response Rate (ORR), and Overall Survival (OS). Patients were randomized (2:1) to receive either 50 mg SUTENT or placebo orally, once daily, on Schedule 4/2 until disease progression or withdrawal from the study for another reason. Treatment was unblinded at the time of disease progression. Patients randomized to placebo were then offered crossover to open-label SUTENT, and patients randomized to SUTENT were permitted to continue treatment per investigator judgment.
At the time of a pre-specified interim analysis, the intent-to-treat (ITT) population included 312 patients. Two-hundred seven (207) patients were randomized to the SUTENT arm, and 105 patients were randomized to the placebo arm. Demographics were comparable between the SUTENT and placebo groups with regard to age (69% vs 72% <65 years for SUTENT vs. placebo, respectively), gender (Male: 64% vs. 61%), race (White: (88% both arms, Asian: 5% both arms, Black: 4% both arms, remainder not reported), and Performance Status (ECOG 0: 44% vs. 46%, ECOG 1: 55% vs. 52%, and ECOG 2: 1 vs. 2%). Prior treatment included surgery (94% vs. 93%) and radiotherapy (8% vs. 15%). Outcome of prior imatinib treatment was also comparable between arms with intolerance (4% vs. 4%), progression within 6 months of starting treatment (17% vs. 16%), or progression beyond 6 months (78% vs. 80%) balanced.
The planned interim efficacy and safety analysis was performed after 149 TTP events had occurred. There was a statistically significant advantage for SUTENT over placebo in TTP, meeting the primary endpoint. Efficacy results are summarized in Table 7 and the Kaplan-Meier curve for TTP is in Figure 1.
| Efficacy Parameter | SUTENT (n=207) |
Placebo (n=105) |
P-value (log-rank test) | HR (95% CI) |
|---|---|---|---|---|
| CI=Confidence interval, HR=Hazard ratio, PR=Partial response | ||||
Time to Tumor Progression |
27.3 (16.0, 32.1) |
6.4 (4.4, 10.0) |
<0.0001 |
0.33 (0.23, 0.47) |
Progression-free Survival |
24.1 (11.1, 28.3) |
6.0 (4.4, 9.9) |
<0.0001 | 0.33 (0.24, 0.47) |
| Objective Response Rate (PR) [%, (95% CI)] | 6.8 (3.7, 11.1) |
0 | 0.006 |
|
Figure 1. Kaplan-Meier Curve of TTP in GIST Study A (Intent-to-Treat Population)
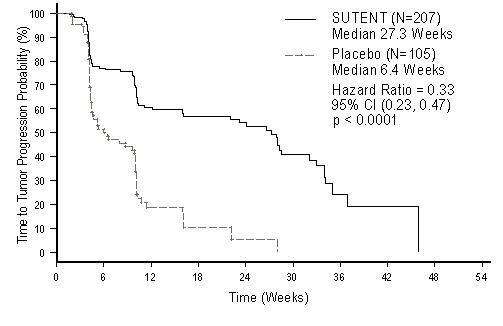
The final ITT population enrolled in the double-blind treatment phase of the study included 243 patients randomized to the SUTENT arm and 118 patients randomized to the placebo arm. After the primary endpoint was met at the interim analysis, the study was unblinded, and patients on the placebo arm were offered open-label SUTENT treatment. Ninety-nine of the patients initially randomized to placebo crossed over to receive SUTENT in the open-label treatment phase. At the protocol specified final analysis of OS, the median OS was 72.7 weeks for the SUTENT arm and 64.9 weeks for the placebo arm [HR= 0.876, 95% CI (0.679, 1.129)].
Study B
Study B was an open-label, multi-center, single-arm, dose-escalation study conducted in patients with GIST following progression on or intolerance to imatinib. Following identification of the recommended Phase 2 regimen (50 mg once daily on Schedule 4/2), 55 patients in this study received the 50 mg dose of SUTENT on treatment Schedule 4/2. Partial responses were observed in 5 of 55 patients [9.1% PR rate, 95% CI (3.0, 20.0)].
14.2 Renal Cell Carcinoma
Treatment-Naïve RCC
A multi-center, international randomized study comparing single-agent SUTENT with IFN-α was conducted in patients with treatment-naïve RCC. The objective was to compare Progression-Free Survival (PFS) in patients receiving SUTENT versus patients receiving IFN-α. Other endpoints included Objective Response Rate (ORR), Overall Survival (OS) and safety. Seven hundred fifty (750) patients were randomized (1:1) to receive either 50 mg SUTENT once daily on Schedule 4/2 or to receive IFN-α administered subcutaneously at 9 MIU three times a week. Patients were treated until disease progression or withdrawal from the study.
The ITT population included 750 patients, 375 randomized to SUTENT and 375 randomized to IFN-α. Demographics were comparable between the SUTENT and IFN-α groups with regard to age (59% vs. 67% <65 years for SUTENT vs. IFN-α, respectively), gender (Male: 71% vs. 72%), race (White: 94% vs. 91%, Asian: 2% vs. 3%, Black: 1% vs. 2%, remainder not reported), and Performance Status (ECOG 0: 62% vs. 61%, ECOG 1: 38% each arm, ECOG 2: 0 vs. 1%). Prior treatment included nephrectomy (91% vs. 89%) and radiotherapy (14% each arm). The most common site of metastases present at screening was the lung (78% vs. 80%, respectively), followed by the lymph nodes (58% vs. 53%, respectively) and bone (30% each arm); the majority of the patients had multiple (2 or more) metastatic sites at baseline (80% vs. 77%, respectively).
There was a statistically significant advantage for SUTENT over IFN-α in the endpoint of PFS (see Table 8 and Figure 2). In the pre-specified stratification factors of LDH (>1.5 ULN vs. ≤1.5 ULN), ECOG performance status (0 vs. 1), and prior nephrectomy (yes vs. no), the hazard ratio favored SUTENT over IFN-α. The ORR was higher in the SUTENT arm (see Table 8).
| Efficacy Parameter | SUTENT (n=375) |
IFN-α (n=375) |
P-value (log-rank test) | HR (95% CI) |
|---|---|---|---|---|
| CI=Confidence interval, NA=Not applicable | ||||
Progression-Free Survival |
47.3 (42.6, 50.7) |
22.0 (16.4, 24.0) |
<0.000001 |
0.415 (0.320, 0.539) |
Objective Response Rate |
27.5 (23.0, 32.3) |
5.3 (3.3, 8.1) |
<0.001 |
NA |
Figure 2. Kaplan-Meier Curve of PFS in Treatment-Naïve RCC Study (Intent-to-Treat Population)
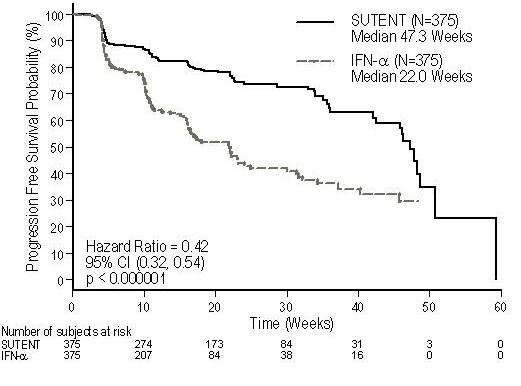
At the protocol-specified final analysis of OS, the median OS was 114.6 weeks for the SUTENT arm and 94.9 weeks for the IFN-α arm [HR= 0.821, 95% CI (0.673, 1.001)]. The median OS for the IFN-α arm includes 25 patients who discontinued IFN-α treatment because of disease progression and crossed over to treatment with SUTENT as well as 121 patients (32%) on the IFN-α arm who received post-study cancer treatment with SUTENT.
Cytokine-Refractory RCC
The use of single agent SUTENT in the treatment of cytokine-refractory RCC was investigated in two single-arm, multi-center studies. All patients enrolled into these studies experienced failure of prior cytokine-based therapy. In Study 1, failure of prior cytokine therapy was based on radiographic evidence of disease progression defined by RECIST or World Health Organization (WHO) criteria during or within 9 months of completion of 1 cytokine therapy treatment (IFN-α, interleukin-2, or IFN-α plus interleukin-2; patients who were treated with IFN-α alone must have received treatment for at least 28 days). In Study 2, failure of prior cytokine therapy was defined as disease progression or unacceptable treatment-related toxicity. The endpoint for both studies was ORR. Duration of Response (DR) was also evaluated.
One hundred six patients (106) were enrolled into Study 1, and 63 patients were enrolled into Study 2. Patients received 50 mg SUTENT on Schedule 4/2. Therapy was continued until the patients met withdrawal criteria or had progressive disease. The baseline age, gender, race and ECOG performance statuses of the patients were comparable between Studies 1 and 2. Approximately 86–94% of patients in the two studies were White. Men comprised 65% of the pooled population. The median age was 57 years and ranged from 24 to 87 years in the studies. All patients had an ECOG performance status <2 at the screening visit.
The baseline malignancy and prior treatment history of the patients were comparable between Studies 1 and 2. Across the two studies, 95% of the pooled population of patients had at least some component of clear-cell histology. All patients in Study 1 were required to have a histological clear-cell component. Most patients enrolled in the studies (97% of the pooled population) had undergone nephrectomy; prior nephrectomy was required for patients enrolled in Study 1. All patients had received one previous cytokine regimen. Metastatic disease present at the time of study entry included lung metastases in 81% of patients. Liver metastases were more common in Study 1 (27% vs. 16% in Study 2) and bone metastases were more common in Study 2 (51% vs. 25% in Study 1); 52% of patients in the pooled population had at least 3 metastatic sites. Patients with known brain metastases or leptomeningeal disease were excluded from both studies.
The ORR and DR data from Studies 1 and 2 are provided in Table 9. There were 36 PRs in Study 1 as assessed by a core radiology laboratory for an ORR of 34.0% (95% CI 25.0, 43.8). There were 23 PRs in Study 2 as assessed by the investigators for an ORR of 36.5% (95% CI 24.7, 49.6). The majority (>90%) of objective disease responses were observed during the first four cycles; the latest reported response was observed in Cycle 10. DR data from Study 1 is premature as only 9 of 36 patients (25%) responding to treatment had experienced disease progression or died at the time of the data cutoff.
| Efficacy Parameter | Study 1 (N=106) |
Study 2 (N=63) |
|---|---|---|
| CI=Confidence interval | ||
| Objective Response Rate [%, (95% CI)] | 34.0 (25.0, 43.8) |
36.5 (24.7, 49.6) |
| Duration of Response (DR) [median, weeks (95% CI)] |
 (42.0,  |
54 (34.3, 70.1) |
14.3 Pancreatic Neuroendocrine Tumors
The Phase 3 study was a multi-center, international, randomized, double-blind placebo-controlled study of single-agent SUTENT conducted in patients with unresectable pNET. Patients were required to have documented RECIST-defined disease progression within the prior 12 months and were randomized (1:1) to receive either 37.5 mg SUTENT (n=86) or placebo (n=85) once daily without a scheduled off-treatment period. The primary objective was to compare Progression-Free Survival (PFS) in patients receiving SUTENT versus patients receiving placebo. Other endpoints included Overall Survival (OS), Objective Response Rate (ORR), and safety. Use of somatostatin analogs was allowed in the study.
Demographics were comparable between the SUTENT and placebo groups. Additionally, 49% of SUTENT patients had non-functioning tumors vs 52% of placebo patients, and 92% patients in both arms had liver metastases. A total of 66% of SUTENT patients received prior systemic therapy compared with 72% of placebo patients and 35% of SUTENT patients had received somatostatin analogs compared with 38% of placebo patients. Patients were treated until disease progression or withdrawal from the study. Upon disease progression, or study closure, patients were offered access to SUTENT in a separate extension study.
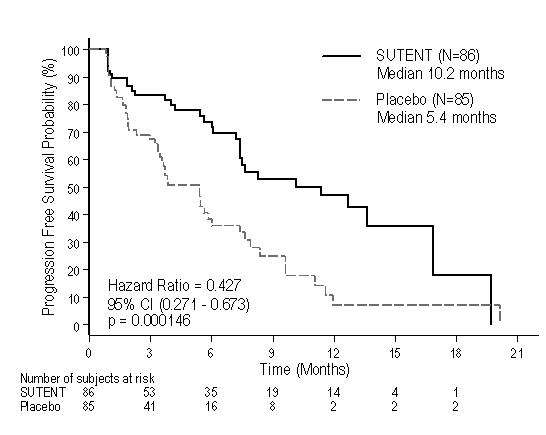
As recommended by the Independent Data Monitoring Committee, the study was terminated prematurely prior to the pre-specified interim analysis. This may have led to an overestimate of the magnitude of PFS effect. A clinically significant improvement for SUTENT over placebo in PFS was seen by both investigator and independent assessment. A hazard ratio favoring SUTENT was observed in all subgroups of baseline characteristics evaluated. OS data were not mature at the time of the analysis. There were 9 deaths in the SUTENT arm and 21 deaths in the placebo arm. A statistically significant difference in ORR favoring SUTENT over placebo was observed. Efficacy results are summarized in Table 10 and the Kaplan-Meier curve for PFS is in Figure 3.
| Efficacy Parameter | SUTENT (n=86) |
Placebo (n=85) |
P-value | HR (95% CI) |
|---|---|---|---|---|
| CI=Confidence interval, HR=Hazard ratio, NA=Not applicable | ||||
| Progression-Free Survival [median, months (95% CI)] | 10.2 (7.4, 16.9) |
5.4 (3.4, 6.0) |
0.000146 |
0.427 (0.271, 0.673) |
| Objective Response Rate [%, (95% CI)] | 9.3 (3.2, 15.4) |
0 | 0.0066 |
NA |
Figure 3. Kaplan-Meier Curve of PFS in the pNET Phase 3 Study
16 HOW SUPPLIED/STORAGE AND HANDLING
12.5 mg Capsules
Hard gelatin capsule with orange cap and orange body, printed with white ink "Pfizer" on the cap, "STN 12.5 mg" on the body; available in:
Bottles of 28: NDC 0069-0550-38
25 mg Capsules
Hard gelatin capsule with caramel cap and orange body, printed with white ink "Pfizer" on the cap, "STN 25 mg" on the body; available in:
Bottles of 28: NDC 0069-0770-38
50 mg Capsules
Hard gelatin capsule with caramel cap and caramel body, printed with white ink "Pfizer" on the cap, "STN 50 mg" on the body; available in:
Bottles of 28: NDC 0069-0980-38
Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling.
17.1 Gastrointestinal Disorders
Gastrointestinal disorders such as diarrhea, nausea, stomatitis, dyspepsia, and vomiting were the most commonly reported gastrointestinal events occurring in patients who received SUTENT. Supportive care for gastrointestinal adverse events requiring treatment may include anti-emetic or anti-diarrheal medication.
17.2 Skin Effects
Skin discoloration possibly due to the drug color (yellow) occurred in approximately one third of patients. Patients should be advised that depigmentation of the hair or skin may occur during treatment with SUTENT. Other possible dermatologic effects may include dryness, thickness or cracking of skin, blister or rash on the palms of the hands and soles of the feet.
17.3 Other Common Events
Other commonly reported adverse events included fatigue, high blood pressure, bleeding, swelling, mouth pain/irritation and taste disturbance.
17.4 Musculoskeletal Disorders
Prior to treatment with SUTENT, a dental examination and appropriate preventive dentistry should be considered. In patients being treated with SUTENT, who have previously received or are receiving bisphosphonates, invasive dental procedures should be avoided, if possible.
17.5 Concomitant Medications
Patients should be advised to inform their health care providers of all concomitant medications, including over-the-counter medications and dietary supplements [see Drug Interactions (7)].
This product's label may have been updated. For full prescribing information, please visit www.pfizer.com.

LAB-0317-18.0
MEDICATION GUIDE
SUTENT (su TENT)
(sunitinib malate)
capsules
Read the Medication Guide that comes with SUTENT before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or treatment. If you have any questions about SUTENT, ask your healthcare provider or pharmacist.
What is the most important information I should know about SUTENT?
SUTENT can cause serious liver problems, including death.
-
Tell your healthcare provider right away if you develop any of the following signs and symptoms of liver problems during treatment with SUTENT:
- itching
- yellow eyes or skin,
- dark urine, and
- pain or discomfort in the right upper stomach area.
- Your healthcare provider should do blood tests to check your liver function before you start taking SUTENT and during treatment.
What is SUTENT?
SUTENT is a prescription medicine used to treat people with:
- a rare cancer of the stomach, bowel, or esophagus called GIST (gastrointestinal stromal tumor) and when:
- the medicine Gleevec® (imatinib mesylate) did not stop the cancer from growing, or
- you cannot take Gleevec®.
- advanced kidney cancer (advanced renal cell carcinoma or RCC).
- a type of pancreatic cancer known as pancreatic neuroendocrine tumors (pNET), that has progressed and cannot be treated with surgery.
It is not known if SUTENT is safe and effective in children.
What should I tell my healthcare provider before taking SUTENT?
Before taking SUTENT tell your healthcare provider if you:
- have any heart problems
- have high blood pressure
- have thyroid problems
- have kidney function problems (other than cancer)
- have liver problems
- have any bleeding problem
- have seizures
- have or have had pain in the mouth, teeth or jaw, swelling or sores inside the mouth, numbness or a feeling of heaviness in the jaw, or loosening of a tooth
- have any other medical conditions
- are pregnant, could be pregnant or plan to become pregnant. SUTENT may harm an unborn baby. You should not become pregnant while taking SUTENT. Tell your healthcare provider right away if you become pregnant while taking SUTENT.
- are breastfeeding or plan to breastfeed. You and your healthcare provider should decide if you will take SUTENT or breastfeed. You should not do both.
Tell all of your healthcare providers and dentists that you are taking SUTENT. They should talk to the healthcare provider who prescribed SUTENT for you, before you have any surgery, or medical or dental procedure.
Tell your healthcare provider about all the medicines you take, including prescription medicines and non-prescription medicines, vitamins, and herbal supplements. Using SUTENT with certain other medicines can cause serious side effects.
You may have an increased risk of severe jaw bone problems (osteonecrosis) if you take SUTENT and a bisphosphonate medicine. Especially tell your healthcare provider if you are taking or have taken Actonel, Aredia, Boniva, Didronel, Fosamax, Reclast, Skelid or Zometa.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. Talk with your healthcare provider before starting any new medicines.
How should I take SUTENT?
- Take SUTENT exactly the way your healthcare provider tells you.
- Take SUTENT 1 time each day with or without food.
- If you take SUTENT for GIST or RCC, you will usually take your medicine for 4 weeks (28 days) and then stop for 2 weeks (14 days). This is 1 cycle of treatment. You will repeat this cycle for as long as your healthcare provider tells you to.
- If you take SUTENT for pNET, take it one time each day until your healthcare provider tells you to stop.
- Do not open the SUTENT capsules.
- Do not drink grapefruit juice or eat grapefruit during your treatment with SUTENT. They may cause you to have too much SUTENT in your body.
- Your healthcare provider may do blood tests before each cycle of treatment.
- If you miss a dose, take it as soon as you remember. Do not take it if it is close to your next dose. Just take the next dose at your regular time. Do not take more than 1 dose of SUTENT at a time. Tell your healthcare provider about any missed dose.
- Call your healthcare provider right away, if you take too much SUTENT.
What are possible side effects of SUTENT?
SUTENT may cause serious side effects including:
- See "What is the most important information I should know about SUTENT?"
- Heart problems. Heart problems may include heart failure and heart muscle problems (cardiomyopathy) that can lead to death. Tell your healthcare provider if you feel very tired, are short of breath, or have swollen feet and ankles.
- Abnormal heart rhythm changes. Your healthcare provider may do electrocardiograms and blood tests to watch for these problems during your treatment with SUTENT. Tell your healthcare provider if you feel dizzy, faint, or have abnormal heartbeats while taking SUTENT.
- High blood pressure. Your healthcare provider may check your blood pressure during treatment with SUTENT. Your healthcare provider may prescribe medicine for you to treat high blood pressure, if needed.
-
Bleeding sometimes leading to death. Tell your healthcare provider right away if you have any of these symptoms or a serious bleeding problem during treatment with SUTENT.
- painful, swollen stomach (abdomen)
- vomiting blood
- black, sticky stools
- bloody urine
- headache or change in your mental status
Your healthcare provider can tell you other symptoms to watch for. -
Jaw-bone problems (osteonecrosis)
Severe jaw bone problems may happen when you take SUTENT. Your healthcare provider should examine your mouth before you start SUTENT. Your healthcare provider may tell you to see your dentist before you start SUTENT. - Tumor lysis syndrome (TLS). TLS is caused by the fast breakdown of cancer cells and may lead to death. TLS may cause you to have nausea, shortness of breath, irregular heartbeat, clouding of urine and tiredness associated with abnormal laboratory test results (high potassium, uric acid and phosphorous levels and low calcium levels in the blood) that can lead to changes in kidney function and acute kidney failure. Your healthcare provider may do blood tests to check you for TLS.
-
Hormone problems, including thyroid and adrenal gland problems. Your healthcare provider may do tests to check your thyroid and adrenal gland function during SUTENT treatment. Tell your doctor if you have any of the following signs and symptoms during treatment with SUTENT:
- tiredness that worsens and does not go away
- loss of appetite
- heat intolerance
- feeling nervous or agitated, tremors
- sweating
- nausea or vomiting
- diarrhea
- fast heart rate
- weight gain or weight loss
- feeling depressed
- irregular menstrual periods or no menstrual periods
- headache
- hair loss
Common side effects of SUTENT include:
|
|
Call your healthcare provider if you have any swelling or bleeding during treatment with SUTENT.
These are not all the possible side effects of SUTENT. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store SUTENT?
- Store SUTENT at room temperature, between 59°F to 86°F (15°C to 30°C).
Keep SUTENT and all medicines out of the reach of children.
General information about SUTENT
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use SUTENT for a condition for which it was not prescribed. Do not give SUTENT to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide gives the most important information about SUTENT. For more information about SUTENT, talk with your healthcare provider or pharmacist. You can ask your healthcare provider or pharmacist for information about SUTENT that is written for health professionals.
For more information go to www.SUTENT.com or call 1-877-5-SUTENT.
What are the ingredients in SUTENT?
Active ingredient: sunitinib malate
Inactive ingredients: mannitol, croscarmellose sodium, povidone (K-25), magnesium stearate Orange gelatin capsule shell: titanium dioxide, red iron oxide Caramel gelatin capsule shell: titanium dioxide, red iron oxide, yellow iron oxide, black iron oxide White printing ink: shellac, propylene glycol, sodium hydroxide, povidone, titanium dioxide
This Medication Guide has been approved by the U.S. Food and Drug Administration.

Gleevec® is a registered trademark of Novartis Pharmaceuticals Corp
LAB-0361-6.0
August 2013
PRINCIPAL DISPLAY PANEL - 12.5 mg Bottle Label
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0069-0550-38
28 Capsules
Rx only
Sutent
®
(sunitinib malate)
12.5 mg*
Distributed by
Pfizer Labs
Division of Pfizer Inc, NY, NY 10017

PRINCIPAL DISPLAY PANEL - 25 mg Bottle Label
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0069-0770-38
28 Capsules
Rx only
Sutent
®
(sunitinib malate)
25 mg*
Distributed by
Pfizer Labs
Division of Pfizer Inc, NY, NY 10017

PRINCIPAL DISPLAY PANEL - 50 mg Bottle Label
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0069-0980-38
28 Capsules
Rx only
Sutent
®
(sunitinib malate)
50 mg*
Distributed by
Pfizer Labs
Division of Pfizer Inc, NY, NY 10017

SUTENTSunitinib malate CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SUTENTSunitinib malate CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SUTENTSunitinib malate CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||