Tamsulosin Hydrochloride
Sun Pharmaceutical Industries Limited
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use tamsulosin hydrochloride safely and effectively. See full prescribing information for tamsulosin hydrochloride capsules. Tamsulosin Hydrochloride Capsules USP, 0.4 mgInitial U.S. Approval: 1997 RECENT MAJOR CHANGES5.5INDICATIONS AND USAGE Tamsulosin hydrochloride is an alpha1 adrenoceptor antagonist indicated for treatment of the signs and symptoms of benign prostatic hyperplasia (1) Tamsulosin hydrochloride capsules are not indicated for the treatment of hypertension (1) DOSAGE AND ADMINISTRATION 0.4 mg once daily taken approximately one-half hour following the same meal each day (2) Can be increased to 0.8 mg once daily for patients who fail to respond to the 0.4 mg dose after 2 to 4 weeks of dosing (2) If discontinued or interrupted for several days, therapy should start again with the 0.4 mg once-daily dose (2) DOSAGE FORMS AND STRENGTHS Capsules: 0.4 mg (3) CONTRAINDICATIONS Contraindicated in patients known to be hypersensitive to tamsulosin hydrochloride or any component of tamsulosin hydrochloride capsules (4, 6.2) WARNINGS AND PRECAUTIONS Advise patients about the possibility of symptoms related to postural hypotension and to avoid situations where injury could result should syncope occur (5.1) Should not be used in combination with strong inhibitors of CYP3A4. Use with caution in combination with moderate inhibitors of CYP3A4, with strong or moderate inhibitors of CYP2D6, in patients known to be CYP2D6 poor metabolizers, or in combination with other cytochrome P450 inhibitors. (5.2, 7.1, 12.3) Should not be used in combination with other alpha adrenergic blocking agents (5.2, 7.2, 12.3) Exercise caution with concomitant administration of warfarin (5.2, 7.4, 12.3) Advise patients about the possibility and seriousness of priapism (5.3) Intraoperative Floppy Iris Syndrome has been observed during cataract surgery in some patients. Advise patients considering cataract surgery to tell their ophthalmologist that they have taken tamsulosin hydrochloride capsules. (5.5) Advise patients to be screened for the presence of prostate cancer prior to treatment and at regular intervals afterwards (5.4) Side Effects The most common adverse events (≥2% of patients and at a higher incidence than placebo) with the 0.4 mg dose or 0.8 mg dose were headache, dizziness, rhinitis, infection, abnormal ejaculation, asthenia, back pain, diarrhea, pharyngitis, chest pain, cough increased, somnolence, nausea, sinusitis, insomnia, libido decreased, tooth disorder, and blurred vision (6.1) To report SUSPECTED ADVERSE REACTIONS, contact CARACO Pharmaceutical Laboratories Ltd. at 1-800-818-4555, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS Tamsulosin hydrochloride capsules 0.4 mg should not be used with strong inhibitors of CYP3A4 (e.g., ketoconazole). Tamsulosin hydrochloride capsules should be used with caution in combination with moderate inhibitors of CYP3A4 (e.g., erythromycin), in combination with strong (e.g., paroxetine) or moderate (e.g., terbinafine) inhibitors of CYP2D6, or in patients known to be CYP2D6 poor metabolizers, particularly at a dose higher than 0.4 mg (e.g., 0.8 mg). (5.2, 7.1, 12.3) Concomitant use of PDE5 inhibitors with tamsulosin can potentially cause symptomatic hypotension (5.2, 7.3, 12.3) USE IN SPECIFIC POPULATIONS Pediatric Use: Not indicated for use in pediatric populations (8.4, 12.3) Geriatric Use: No overall differences in efficacy or safety vs younger patients, but greater sensitivity of some older adults cannot be ruled out (8.5, 12.3) Renal Impairment: Has not been studied in patients with end-stage renal disease (8.6, 12.3) Hepatic Impairment: Has not been studied in patients with severe hepatic impairment (8.7, 12.3)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 TAMSULOSIN HYDROCHLORIDE INDICATIONS AND USAGE
- 2 TAMSULOSIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 TAMSULOSIN HYDROCHLORIDE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 TAMSULOSIN HYDROCHLORIDE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 TAMSULOSIN HYDROCHLORIDE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PRINCIPAL DISPLAY PANEL-Label
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
see Clinical Studies (14)
2 DOSAGE AND ADMINISTRATION
see Warnings and Precautions (5.2)
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
see Adverse Reactions (6.2).
5 WARNINGS AND PRECAUTIONS
5.1 Orthostasis
see Adverse Reactions (6.1)see Patient Counseling Information (17.1)
5.2 Drug Interactions
see Drug Interactions (7.1) and Clinical Pharmacology (12.3)see Drug Interactions (7.1) and Clinical Pharmacology (12.3)
see Drug Interactions (7.1) and Clinical Pharmacology (12.3)
see Drug Interactions (7.2) and Clinical Pharmacology (12.3)
see Drug Interactions (7.3) and Clinical Pharmacology (12.3)
see Drug Interactions (7.4) and Clinical Pharmacology (12.3).
5.3 Priapism
1see Patient Counseling Information (17.2)
5.4 Screening for Prostate Cancer
see Patient Counseling Information (17.3)
5.5 Intraoperative Floppy Iris Syndrome
Intraoperative1see Adverse Reactions (6.2)1111
IFIS1
5.6 Sulfa Allergy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
| BODY SYSTEM/ADVERSE EVENT | TAMSULOSIN HYDROCHLORIDE CAPSULES GROUPS | PLACEBO n=493 |
|
|---|---|---|---|
| 0.4 mg n=502 |
0.8 mg n=492 |
||
|
BODY AS WHOLE
|
|||
| Headache |
97 (19.3%) |
104 (21.1%) |
99 (20.1%) |
Infection |
45 (9%) |
53 (10.8%) |
37 (7.5%) |
| Asthenia |
39 (7.8%) |
42 (8.5%) |
27 (5.5%) |
| Back pain |
35 (7%) |
41 (8.3%) |
27 (5.5%) |
| Chest pain |
20 (4%) |
20 (4.1%) |
18 (3.7%) |
|
NERVOUS SYSTEM
|
|||
| Dizziness |
75 (14.9%) |
84 (17.1%) |
50 (10.1%) |
| Somnolence |
15 (3%) |
21 (4.3%) |
8 (1.6%) |
| Insomnia |
12 (2.4%) |
7 (1.4%) |
3 (0.6%) |
| Libido decreased |
5 (1%) |
10 (2%) |
6 (1.2%) |
|
RESPIRATORY SYSTEM
|
|||
Rhinitis |
66 (13.1%) |
88 (17.9%) |
41 (8.3%) |
| Pharyngitis |
29 (5.8%) |
25 (5.1%) |
23 (4.7%) |
| Cough increased |
17 (3.4%) |
22 (4.5%) |
12 (2.4%) |
| Sinusitis |
11 (2.2%) |
18 (3.7%) |
8 (1.6%) |
|
DIGESTIVE SYSTEM
|
|||
| Diarrhea |
31 (6.2%) |
21 (4.3%) |
22 (4.5%) |
| Nausea |
13 (2.6%) |
19 (3.9%) |
16 (3.2%) |
| Tooth disorder |
6 (1.2%) |
10 (2%) |
7 (1.4%) |
|
UROGENITAL SYSTEM
|
|||
| Abnormal ejaculation |
42 (8.4%) |
89 (18.1%) |
1 (0.2%) |
|
SPECIAL SENSES
|
|||
| Blurred vision |
1 (0.2%) |
10 (2%) |
2 (0.4%) |
see Warnings and Precautions (5.1)
Abnormal Ejaculation
Laboratory Tests
6.2 Postmarketing Experience
1see Warnings and Precautions (5.5)
7 DRUG INTERACTIONS
7.1 Cytochrome P450 Inhibition
Strong and Moderate Inhibitors of CYP3A4 or CYP2D6
maxsee Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)
maxsee Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)
see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)
see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)
Cimetidine
see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)
7.2 Other Alpha Adrenergic Blocking Agents
see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)
7.3 PDE5 Inhibitors
see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)
7.4 Warfarin
in vitro in vivosee Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)
7.5 Nifedipine, Atenolol, Enalapril
see Clinical Pharmacology (12.3)
7.6 Digoxin and Theophylline
see Clinical Pharmacology (12.3)
7.7 Furosemide
maxsee Clinical Pharmacology (12.3)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects, Pregnancy Category B.
8.3 Nursing Mothers
8.4 Pediatric Use
®
8.5 Geriatric Use
see Clinical Pharmacology (12.3)
8.6 Renal Impairment
cr 2see Clinical Pharmacology (12.3)
8.7 Hepatic Impairment
see Clinical Pharmacology (12.3)
10 OVERDOSAGE
see Warnings and Precautions (5.1) and Adverse Reactions (6.1),
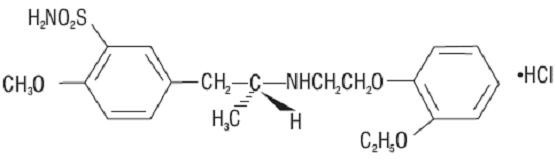
11 DESCRIPTION
1A
Ro
202825
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
1
1111A1B1D11A
12.2 Pharmacodynamics
see Use in Specific Populations (8.4) and Clinical Studies (14)
12.3 Pharmacokinetics
Absorption
Effect of Food
maxmax
Figure 1 Mean Plasma Tamsulosin Hydrochloride Concentrations Following Single-Dose Administration of Tamsulosin Hydrochloride Capsules 0.4 mg Under Fasted and Fed Conditions (n=8)

| Pharmacokinetic Parameter | 0.4 mg QD to healthy volunteers; n=23 (age range 18 to 32 years) |
0.8 mg QD to healthy volunteers; n=22 (age range 55 to 75 years) |
|||
|---|---|---|---|---|---|
| Light Breakfast | Fasted | Light Breakfast | High-Fat Breakfast | Fasted | |
| Cmin (ng/mL) |
4 ± 2.6 |
3.8 ± 2.5 |
12.3 ± 6.7 |
13.5 ± 7.6 |
13.3 ± 13.3 |
| Cmax (ng/mL) |
10.1 ± 4.8 |
17.1 ± 17.1 |
29.8 ± 10.3 |
29.1 ± 11 |
41.6 ± 15.6 |
| Cmax/Cmin Ratio |
3.1 ± 1 |
5.3 ± 2.2 |
2.7 ± 0.7 |
2.5 ± 0.8 |
3.6 ± 1.1 |
| Tmax (hours) |
6 |
4 |
7 |
6.6 |
5 |
| T1/2 (hours) |
- |
- |
- |
- |
14.9 ± 3.9 |
| AUCt (ng•hr/mL) |
151 ± 81.5 |
199 ± 94.1 |
440 ± 195 |
449 ± 217 |
557 ± 257 |
max
max
1/2
t
Distribution
1in vitro
Metabolism
see Warnings and Precautions (5.2) and Drug Interactions (7.1)
in vitro
Excretion
Specific Populations
Pediatric Use
see Use in Specific Populations (8.4)
Geriatric (Age) Use
see Use in Specific Populations (8.5)
Renal Impairment
cr2cr2cr2cr2see Use in Specific Populations (8.6)
Hepatic Impairment
see Use in Specific Populations (8.7)
Drug Interactions
Cytochrome P450 Inhibition
Strong and Moderate Inhibitors of CYP3A4 or CYP2D6
maxsee Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)see Warnings and Precautions (5.2) and Drug Interactions (7.1)
maxsee Warnings and Precautions (5.2) and Drug Interactions (7.1)see Warnings and Precautions (5.2) and Drug Interactions (7.1)
see Warnings and Precautions (5.2) and Drug Interactions (7.1)
see Warnings and Precautions (5.2) and Drug Interactions (7.1)
Cimetidine
see Warnings and Precautions (5.2) and Drug Interactions (7.1)
Other Alpha Adrenergic Blocking Agents
see Warnings and Precautions (5.2) and Drug Interactions (7.2)
PDE5 Inhibitors
see Warnings and Precautions (5.2) and Drug Interactions (7.3)
Warfarin
in vitro in vivosee Warnings and Precautions (5.2) and Drug Interactions (7.4)
Nifedipine, Atenolol, Enalapril
see Drug Interactions (7.5)
Digoxin and Theophylline
see Drug Interactions (7.6)
Furosemide
maxsee Drug Interactions (7.7)
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitroin vivo
14 CLINICAL STUDIES
| Total AUA Symptom Score | Peak Urine Flow Rate | |||
|---|---|---|---|---|
| Mean Baseline Value | Mean Change | Mean Baseline Value | Mean Change | |
|
* Statistically significant difference from placebo (p-value ≤0.05; Bonferroni-Holm multiple test procedure). ** Total AUA Symptom Scores ranged from 0 to 35. † Peak urine flow rate measured 4 to 8 hours post dose at Week 13. ‡ Peak urine flow rate measured 24 to 27 hours post dose at Week 13. Week 13: For patients not completing the 13-week study, the last observation was carried forward. |
||||
|
Study 1
†
|
||||
| Tamsulosin hydrochloride capsules 0.8 mg once daily |
19.9 ± 4.9 n=247 |
-9.6* ± 6.7 n=237 |
9.57 ± 2.51 n=247 |
1.78* ± 3.35 n=247 |
| Tamsulosin hydrochloride capsules 0.4 mg once daily |
19.8 ± 5 n=254 |
-8.3* ± 6.5 n=246 |
9.46 ± 2.49 n=254 |
1.75* ± 3.57 n=254 |
| Placebo |
19.6 ± 4.9 n=254 |
-5.5 ± 6.6 n=246 |
9.75 ± 2.54 n=254 |
0.52 ± 3.39 n=253 |
|
Study 2
‡
|
||||
| Tamsulosin hydrochloride capsules 0.8 mg once daily |
18.2 ± 5.6 n=244 |
-5.8* ± 6.4 n=238 |
9.96 ± 3.16 n=244 |
1.79* ± 3.36 n=237 |
| Tamsulosin hydrochloride capsules 0.4 mg once daily |
17.9 ± 5.8 n=248 |
-5.1* ± 6.4 n=244 |
9.94 ± 3.14 n=248 |
1.52 ± 3.64 n=244 |
| Placebo |
19.2 ± 6 n=239 |
-3.6 ± 5.7 n=235 |
9.95 ± 3.12 n=239 |
0.93 ± 3.28 n=235 |
Figure 2A Mean Change from Baseline in Total AUA Symptom Score (0 to 35) Study 1
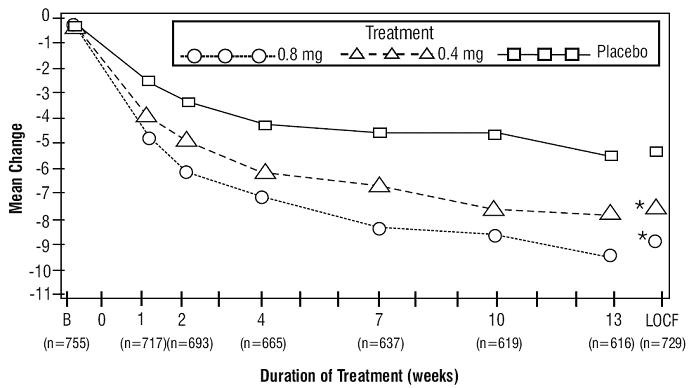
Figure 2B Mean Change from Baseline in Total AUA Symptom Score (0 to 35) Study 2

Figure 3A Mean Increase in Peak Urine Flow Rate (mL/sec) Study 1

Figure 3B Mean Increase in Peak Urine Flow Rate (mL/sec) Study 2

16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (17.6).
17.1 Hypotension
see Warnings and Precautions (5.1)
17.2 Priapism
see Warnings and Precautions (5.3)
17.3 Screening for Prostate Cancer
see Warnings and Precautions (5.4).
17.4 Intraoperative Floppy Iris Syndrome
see Warnings and Precautions (5.5)
17.5 Administration
17.6 FDA-approved Patient Labeling
PATIENT INFORMATION
Tamsulosin Hydrochloride Capsules USP, 0.4 mg
What are tamsulosin hydrochloride capsules?
- Tamsulosin hydrochloride capsules are not for women.
- Tamsulosin hydrochloride capsules are not for children.
What should I tell my doctor before using tamsulosin hydrochloride capsules?
Before taking tamsulosin hydrochloride capsules, tell your doctor about all your medical conditions, including:
- any kidney or liver problems.
- any history of low blood pressure.
- any allergies to sulfa or any other medicines.
- if you are planning to have cataract surgery.
- any prescription medicines, including blood pressure medicines.
- any non-prescription medicines, including vitamins and herbal supplements.
How should I take tamsulosin hydrochloride capsules?
- Take tamsulosin hydrochloride capsules exactly as prescribed by your doctor.
- Do not crush, chew, or open tamsulosin hydrochloride capsules.
- Take tamsulosin hydrochloride capsules one time each day, about 30 minutes after the same meal each day. For example, you may take tamsulosin hydrochloride capsules 30 minutes after dinner each day.
- If you miss a dose of tamsulosin hydrochloride capsules, take it as soon as you remember. If you miss your dose for the whole day, continue with your next dose on your regular schedule. Do not take two doses at the same time.
- If you stop or forget to take tamsulosin hydrochloride capsules for several days, talk with your doctor before starting again.
- If you take more tamsulosin hydrochloride capsules than prescribed, call your doctor right away.
-
Decreased blood pressure when changing positions. Tamsulosin hydrochloride capsules may cause a sudden drop in blood pressure upon standing, especially after the first dose or when changing doses.
Symptoms may include:- fainting
- dizziness
- lightheadedness
Change positions slowly from lying down to sitting up or from a sitting to a standing position until you learn how you react to tamsulosin hydrochloride capsules. If you begin to feel dizzy, sit or lie down until you feel better. If the symptoms are severe or do not improve, call your doctor.
-
Allergic reactions. Make your doctor aware of any allergic reactions you may experience while taking tamsulosin hydrochloride capsules.
Allergic reactions may include:- rash
- itching
- hives
- swelling of face, tongue, or throat
- difficulty breathing
- blistering of the skin
- A painful erection that will not go away. Tamsulosin hydrochloride capsules can cause a painful erection (priapism), which cannot be relieved by having sex. If this happens, get medical help right away. If priapism is not treated, you may not be able to get an erection in the future.
- Eye problems during cataract surgery. During cataract surgery, a condition called intraoperative floppy iris syndrome (IFIS) can happen if you take or have taken tamsulosin hydrochloride capsules. If you need to have cataract surgery, be sure to tell your surgeon if you take or have taken tamsulosin hydrochloride capsules.
- runny nose
- dizziness
- decreased semen
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088, or by visiting www.fda.gov/medwatch.
What should I avoid while taking tamsulosin hydrochloride capsules?
What are the possible side effects of tamsulosin hydrochloride capsules?
How do I store tamsulosin hydrochloride capsules?
Keep tamsulosin hydrochloride capsules and all medicines out of the reach of children.
General information
What are the ingredients in tamsulosin hydrochloride capsules?
- Active Ingredient: tamsulosin hydrochloride
- Inactive Ingredients: microcrystalline cellulose, methacrylic acid copolymer dispersion, hypromellose acetate succinate, triethyl citrate, talc, and sodium lauryl sulfate.
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Industries Ltd.
PRINCIPAL DISPLAY PANEL-Label
NDC 62756-160-83
Tamsulosin Hydrochloride Capsules, USP
0.4 mg
Rx only
30 CAPSULES
SUN PHARMACEUTICAL INDUSTRIES LTD.
PHARMACIST: PLEASE DISPENSE WITH PATIENT INFORMATION LEAFLET

Tamsulosin HydrochlorideTamsulosin Hydrochloride CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!