Tanac
Tanac NO STING LIQUID
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Tanac Uses
- Warnings
- Directions
- Tanac Other information
- Inactive ingredients
- Questions?
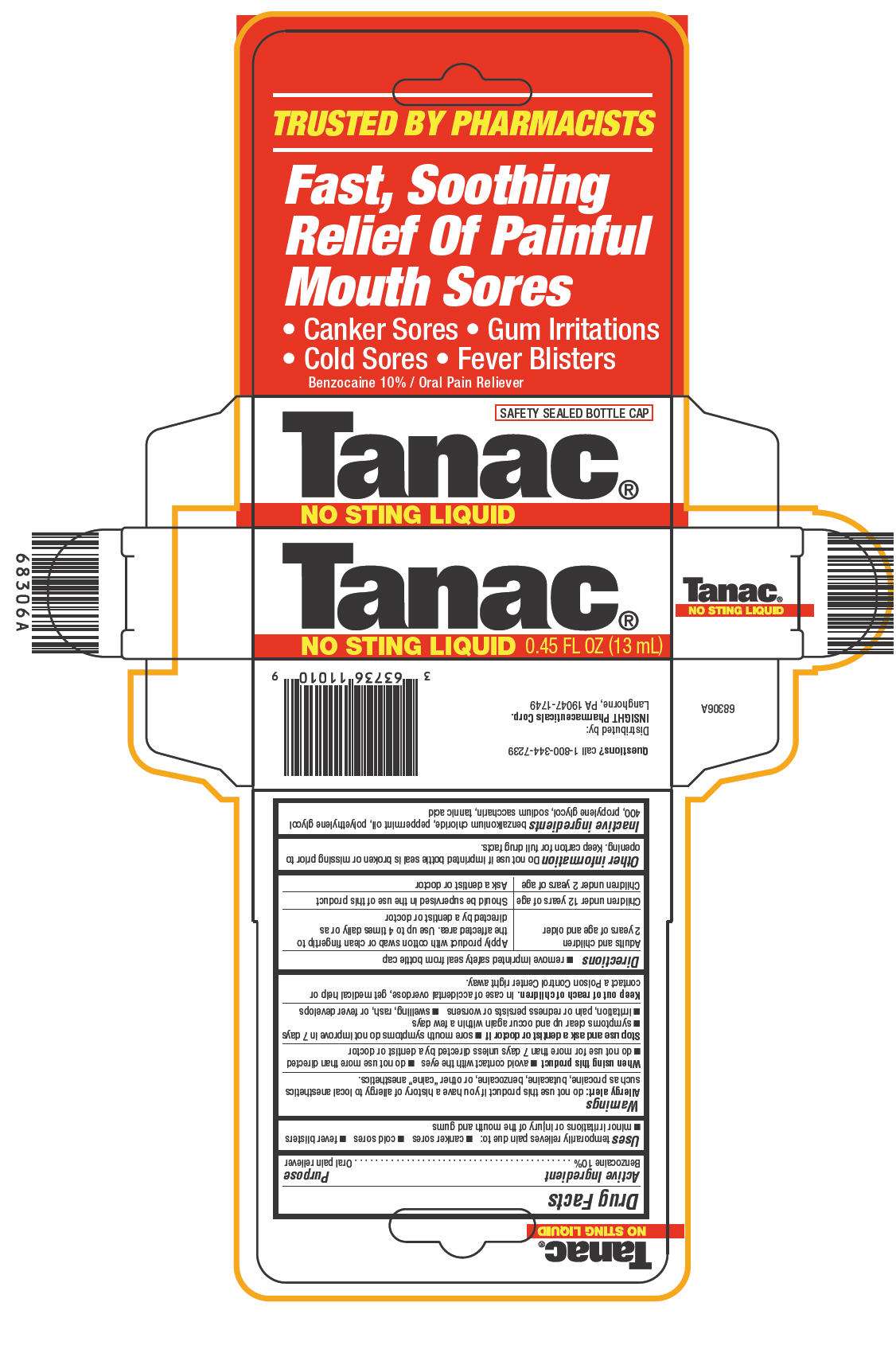
- PRINCIPAL DISPLAY PANEL - 13 mL Bottle Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient
Benzocaine 10%
Purpose
Oral pain reliever
Tanac Uses
temporarily relieves pain due to:
- canker sores
- cold sores
- fever blisters
- minor irritations or injury of the mouth and gums
Warnings
Allergy alert
do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other "caine" anesthetics.
When using this product
- avoid contact with the eyes
- do not use more than directed
- do not use for more than 7 days unless directed by a dentist or doctor
Stop use and ask a dentist or doctor if
- sore mouth symptoms do not improve in 7 days
- symptoms clear up and occur again within a few days
- irritation, pain or redness persists or worsens
- swelling, rash, or fever develops
Keep out of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away.
Directions
- remove imprinted safety seal from bottle cap
| Adults and children 2 years of age and older | Apply product with cotton swab or clean fingertip to the affected area. Use up to 4 times daily or as directed by a dentist or doctor |
| Children under 12 years of age | Should be supervised in the use of this product |
| Children under 2 years of age | Ask a dentist or doctor |
Tanac Other information
Do not use if imprinted bottle seal is broken or missing prior to opening. Keep carton for full drug facts.
Inactive ingredients
benzalkonium chloride, peppermint oil, polyethylene glycol 400, propylene glycol, sodium saccharin, tannic acid
Questions?
call 1-800-344-7239
Distributed by:
INSIGHT Pharmaceuticals Corp.
Langhorne, PA 19047-1749
PRINCIPAL DISPLAY PANEL - 13 mL Bottle Carton
TRUSTED BY PHARMACISTS
Fast, Soothing
Relief Of Painful
Mouth Sores
• Canker Sores • Gum Irritations
• Cold Sores • Fever Blisters
Benzocaine 10% / Oral Pain Reliever
TanacBenzocaine LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||