TC EnrichedFoam
Rubbermaid Commercial Products LLC
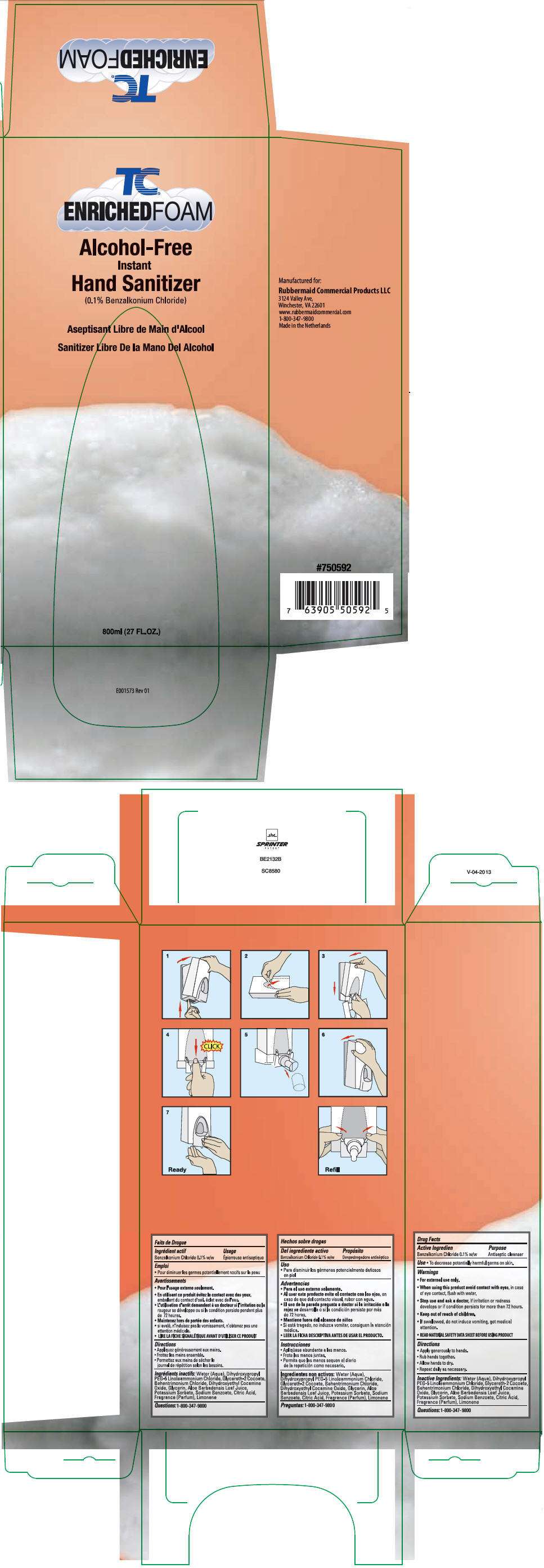
TC ENRICHED FOAM Alcohol-Free Instant Hand Sanitizer
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive Ingredients
- Questions
- PRINCIPAL DISPLAY PANEL - 800 mL Bag Box
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient
Benzalkonium Chloride 0.1% w/w
Purpose
Antiseptic cleanser
Use
- To decrease potentially harmful germs on skin.
Warnings
- For external use only.
- When using this product avoid contact with eyes, in case of eye contact, flush with water.
- Stop use and ask a doctor, if irritation or redness develops or if condition persists for more than 72 hours.
- Keep out of reach of children.
- If swallowed, do not induce vomiting, get medical attention.
- READ MATERIAL SAFETY DATA SHEET BEFORE USING PRODUCT
Directions
- Apply generously to hands.
- Rub hands together.
- Allow hands to dry.
- Repeat daily as necessary.
Inactive Ingredients
Water (Aqua), Dihydroxypropyl PEG-5 Linoleammonium Chloride, Glycereth- 2 Cocoate, Behentrimonium Chloride, Aloe Vera, Glycerin, Fragrance, Dihydroxyethyl Cocamine Oxide
Questions
1- 800-551-5155
info@technicalconcepts.com
Manufactured for:
Technical Concepts LLC,
1301 Allanson Road
Mundelein, Illinois 60060 U.S.A.
1-800-551-5155 • www.technicalconcepts.com
©2007 Technical Concepts.
® and TM : Trademark of Technical Concepts
MADE IN THE NETHERLANDS
PRINCIPAL DISPLAY PANEL - 800 mL Bag Box
TC
®
ENRICHEDFOAM
Alcohol-Free
Instant
Hand Sanitizer
(0.1% Benzalkonium Chloride)
800ml (27 FL.OZ.)
TC EnrichedFoamBenzalkonium Chloride LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||