TechneScan HDP
FULL PRESCRIBING INFORMATION: CONTENTS*
- TECHNESCAN HDP DESCRIPTION
- PHYSICAL CHARACTERISTICS
- EXTERNAL RADIATION
- CLINICAL PHARMACOLOGY
- TECHNESCAN HDP INDICATIONS AND USAGE
- TECHNESCAN HDP CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TECHNESCAN HDP ADVERSE REACTIONS
- TECHNESCAN HDP DOSAGE AND ADMINISTRATION
- RADIATION DOSIMETRY
- PREPARATIONS FOR USE
- UNIT DOSE PREPARATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Rx Only.
Diagnostic – For Intravenous Use
TECHNESCAN HDP DESCRIPTION
TechneScan™ HDP is supplied as a lyophilized powder, packaged under nitrogen in vials for intravenous administration after reconstitution with ADDITIVE-FREE sodium pertechnetate Tc 99m. Each vial contains 3.15 mg oxidronate sodium and 0.258 mg, minimum, stannous chloride (SnCl2•2H2O), 0.297 mg, theoretical, stannous chloride (SnCl2•2H2O) with 0.343 mg, maximum, tin chloride [stannous and stannic] dihydrate as SnCl2•2H2O as active ingredients. In addition, each vial contains 0.84 mg gentisic acid as a stabilizer and 30.0 mg sodium chloride. The pH is adjusted with hydrochloric acid and/or sodium hydroxide. The pH of the reconstituted drug is between 4.0 and 5.5. The contents of the vial are sterile and non-pyrogenic.
The chemical structure of oxidronate sodium is:
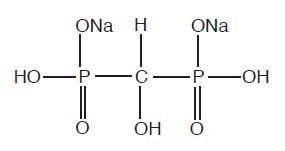
This radiopharmaceutical diagnostic agent, when reconstituted with ADDITIVE-FREE sodium pertechnetate Tc 99m forms a complex of unknown structure.
PHYSICAL CHARACTERISTICS
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours.
Table 1. Principal Radiation Emission Data
|
Radiation |
Mean Percent/ |
Energy |
|
Gamma-2 |
89.07 |
140.5 |
EXTERNAL RADIATION
The specific gamma ray constant for Technetium Tc 99m is 0.78 R/mCi-hr at 1 cm. The first half-value thickness of lead (Pb) for Technetium Tc 99m is 0.017 cm. A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 2. For example, the use of 0.25 cm of Pb will decrease the external radiation exposure by a factor of about 1000.
Table 2. Radiation Attenuation by Lead Shielding
|
Shield Thickness (Pb) cm |
Coefficient of Attenuation |
|
0.017 |
0.5 |
|
0.08 |
10-1 |
|
0.16 |
10-2 |
|
0.25 |
10-3 |
|
0.33 |
10-4 |
To correct for physical decay of this radionuclide, the fractions that remain at selected time intervals after the time of calibration are shown in Table 3.
Table 3. Physical Decay Chart: Technetium Tc 99m Half-Life 6.02 Hours
|
Hours |
Fraction Remaining |
Hours |
Fraction Remaining |
|
0* |
1.000 |
7 |
0.447 |
|
1 |
0.891 |
8 |
0.398 |
|
2 |
0.794 |
9 |
0.355 |
|
3 |
0.708 |
10 |
0.316 |
|
4 |
0.631 |
11 |
0.282 |
|
5 |
0.562 |
12 |
0.251 |
|
6 |
0.501 |
* Calibration time
CLINICAL PHARMACOLOGY
During the 24 hours following injection, Technetium Tc 99m-labeled TechneScan HDP is rapidly cleared from blood and other non-osseous tissues and accumulates in the skeleton and urine in humans. Blood levels are about 10% of the injected dose at one hour post-injection and continue to fall to about 6%, 4% and 3% at 2, 3 and 4 hours, respectively. When measured at 24 hours following its administration, skeletal retention is approximately 50% of the injected dose. TechneScan HDP exhibits its greatest affinity for areas of altered osteogenesis and actively metabolizing bone.
TECHNESCAN HDP INDICATIONS AND USAGE
TechneScan HDP Tc 99m is a diagnostic skeletal imaging agent used to demonstrate areas of altered osteogenesis in adult and pediatric patients.
TECHNESCAN HDP CONTRAINDICATIONS
None known.
WARNINGS
This class of compounds is known to complex cations such as calcium. Particular caution should be used with patients who have, or who may be predisposed to hypocalcemia (i.e., alkalosis).
PRECAUTIONS
General
The components of the kit are sterile and non-pyrogenic. It is essential that the user follow the directions carefully and adhere to strict aseptic procedures during preparation. Sodium pertechnetate Tc 99m solutions which contain an oxidizing agent or saline solutions containing preservatives are not suitable for use in the preparation of TechneScan HDP Tc 99m.
Contents of the vial are intended only for use in the preparation of Technetium Tc 99m Oxidronate and are NOT to be administered directly to the patient. Technetium Tc 99m Oxidronate should be formulated within eight (8) hours prior to clinical use. Optimal imaging results are obtained one to four hours after administration. Technetium Tc 99m Oxidronate as well as other radioactive drugs, must be handled with care, and appropriate safety measures should be used to minimize radiation exposure to the patients consistent with proper patient management and to insure minimum radiation exposure to occupational workers. Radiopharmaceuticals should be used only by physicians who are qualified by specific training in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides. To minimize radiation dose to the bladder, the patients should be encouraged to drink fluids and to void immediately before the examination and as often thereafter as possible for the next four to six hours.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic or mutagenic potential or whether Technetium Tc 99m Oxidronate affects fertility in males and females.
Pregnancy Category C
Animal reproduction studies have not been conducted with Technetium Tc 99m Oxidronate. It is also not known whether Technetium Tc 99m Oxidronate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Technetium Tc 99m Oxidronate should be given to a pregnant woman only if clearly needed. Ideally, examinations using radiopharmaceuticals, especially those elective in nature, of a woman of childbearing capability should be performed during the first few (approximately 10) days following the onset of menses.
Nursing Mothers
Technetium Tc 99m is excreted in human milk during lactation, therefore formula feedings should be substituted for breast feedings.
TECHNESCAN HDP ADVERSE REACTIONS
Some hypersensitivity reactions, as well as nausea and vomiting, have been infrequently associated with Technetium Tc 99m Oxidronate.
TECHNESCAN HDP DOSAGE AND ADMINISTRATION
General Instructions
The recommended adult dose of Technetium Tc 99m-labeled TechneScan HDP is 555 MBq (15 mCi) with a range of 370 to 740 MBq (10 to 20 mCi). The recommended pediatric dose is 7.4 MBq (0.20 mCi)/kg with a range of 7.4 to 13 MBq (0.20 to 0.35 mCi)/kg. The recommended minimum total pediatric dose is 37 MBq (1.0 mCi). The maximum total dose injected into a pediatric or adult patient is 740 MBq (20.0 mCi). The maximum dose of oxidronate sodium should not exceed 2 mg.
Unit dose preparation instructions should be followed for pediatric patients. The radioactivity of each dose should be measured by a suitable radiation calibration system just prior to administration. The dose should be given intravenously by slow injection. For optimal results imaging should be performed 1 to 4 hours post-injection.
RADIATION DOSIMETRY
The estimated absorbed radiation doses from an intravenous injection of Technetium Tc 99m-labeled TechneScan HDP are shown in Table 4.
Table 4. Estimated Absorbed Radiation Doses*
|
Ages |
Newborn |
1 Year Old |
5 Year Old |
10 Year Old |
15 Year Old |
Adult |
||||||
|
Weight (kg) |
3.5 |
12.1 |
20.3 |
33.5 |
55.0 |
70.0 |
||||||
|
Maximum Recommended Dose** |
45.5 MBq (1.2 mCi) |
157.3 MBq (4.2 mCi) |
263.9 MBq (7.1 mCi) |
435.5 MBq (11.7 mCi) |
715.0 MBq (19.3 mCi) |
740.0 MBq (20.0 mCi) |
||||||
|
Tissue |
Estimated Absorbed Radiation Doses |
|||||||||||
|
mGy |
rads |
mGy |
rads |
mGy |
rads |
mGy |
rads |
mGy |
rads |
mGy |
rads |
|
|
Kidneys |
3.0 |
0.30 |
4.2 |
0.42 |
4.0 |
0.40 |
4.4 |
0.44 |
5.2 |
0.52 |
4.4 |
0.44 |
|
Ovaries |
1.5 |
0.15 |
2.5 |
0.25 |
2.4 |
0.24 |
2.6 |
0.26 |
3.0 |
0.30 |
2.4 |
0.24 |
|
Red Marrow |
10.9 |
1.09 |
12.9 |
1.29 |
10.6 |
1.06 |
10.0 |
1.00 |
10.0 |
1.00 |
9.6 |
0.96 |
|
Bone Surfaces |
104.6 |
10.46 |
113.3 |
11.33 |
79.2 |
7.92 |
78.4 |
7.84 |
78.7 |
7.87 |
64.4 |
6.44 |
|
Testes |
1.2 |
0.12 |
2.0 |
0.20 |
1.8 |
0.18 |
1.9 |
0.19 |
2.1 |
0.21 |
1.6 |
0.16 |
|
Bladder Wall |
11.4 |
1.14 |
17.3 |
1.73 |
15.6 |
1.56 |
17.4 |
1.74 |
19.3 |
1.93 |
15.5 |
1.55 |
|
Total Body |
1.8 |
0.18 |
2.7 |
0.27 |
2.6 |
0.26 |
2.7 |
0.27 |
3.0 |
0.30 |
2.5 |
0.25 |
* Based on data in MIRD Dose Estimate Report No. 14. Bladder initially voided at 2.0 hours and then every 4.8 hours thereafter.
**See Dosage and Administration section.
PREPARATIONS FOR USE
All procedures should be conducted using waterproof gloves. Use shielded syringe during transport and administration of Tc 99m solutions.
- Remove plastic disc from TechneScan HDP vial and cleanse top by swabbing with alcohol. Note: If dose is for a single adult patient or for a pediatric patient, see unit dose preparation method below.
- Place vial in lead vial shield. Add 3 to 6 mL of sodium pertechnetate Tc 99m solution and secure with a fitted lead cover. In choosing the amount of Tc 99m radioactivity to be used, the number of doses desired, the activity of each dose [recommended adult dose is 555 MBq (15 mCi) with a range of 370 to 740 MBq (10 to 20 mCi)] and radioactive decay must be taken into account. The recommended maximum amount of Tc 99m radioactivity to be added to the vial is 11.1 gigabecquerels (300 mCi). Note: The contents of the vial are now radioactive. Maintain adequate shielding using the lead vial shield and fitted lead cover during the life of the radioactive preparation.
- Shake the vial gently, for approximately 30 seconds, to assure complete dissolution.
- Record the time, date of preparation and the activity of the Tc 99m labeled TechneScan HDP on the radioassay information label and affix to the vial.
- Use within eight (8) hours of preparation. Refrigeration of the radiolabeled complex is not necessary. Discard unused material in accordance with Nuclear Regulatory Commission or Agreement State regulations pertaining to the disposal of radioactive wastes.
UNIT DOSE PREPARATION
Preparing a dose for a single adult patient or for a pediatric patient
To minimize volume injected and to insure optimum solution concentration, reconstitute the vial contents in 3 to 6 mL of sterile, non-pyrogenic normal saline containing no preservatives. Shake the vial gently for approximately 30 seconds to assure complete dissolution, withdraw and discard all but approximately 1 mL of the solution. Add appropriate amount of sodium pertechnetate Tc 99m for a single adult dose or for one or more pediatric doses and shake gently. Proceed with steps 4 and 5. No more than 1480 MBq (40 mCi) should be added to the vial when preparing multiple pediatric doses. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
HOW SUPPLIED
TechneScan HDP is supplied as a lyophilized powder packaged in vials. Each vial contains 3.15 mg oxidronate sodium and 0.258 mg, minimum, stannous chloride (SnCl2•2H2O), 0.297 mg, theoretical, stannous chloride (SnCl2•2H2O) with 0.343 mg, maximum, tin chloride [stannous and stannic] dihydrate as SnCl2•2H2O. In addition, each vial contains 0.84 mg gentisic acid as a stabilizer and 30.0 mg sodium chloride. Kits containing 5 vials (NDC 0019-9091-20) or 30 vials (NDC 0019-9091-40) are available. The drug can be stored at controlled room temperature 20-25ºC (68-77ºF) both prior to and following reconstitution with ADDITIVE-FREE sodium pertechnetate Tc 99m.
This reagent kit is approved for distribution to persons licensed by the U.S. Nuclear Regulatory Commission to use byproduct material identified in Section 35.200 or under an equivalent license of an Agreement State.
Manufactured by:
Mallinckrodt Inc.
St. Louis, MO 63134 USA
Distributed in Canada by:
tyco Healthcare
Pointe-Claire, QC, Canada H9R 5H8
Estb. Lic. No.: 100689-A
A091I0
Rev 12/2011
Mallinckrodt
PRINCIPAL DISPLAY PANEL
TechneScan™ HDP
Kit for the Preparation of Technetium Tc 99m Oxidronate
Sterile, Non-Pyrogenic, for IV Injection with Sodium Pertechnetate Tc 99m
Rx Only.
Each vial contains 3.15 mg oxidronate sodium and 0.258 mg, minimum, stannous chloride (SnCl2 ● 2H2O), 0.297 mg, theoretical, stannous chloride (SnCl2 ● 2H2O), with 0.343 mg, maximum tin chloride [stannous and stannic] dihydrate as SnCl2 ● 2H2O. In addition, each vial contains 0.84 mg gentisic acid (stabilizer) and 30.0 mg sodium chloride. The pH is adjusted with HCI and/or NaOH. Contents are lyophilized and sealed under nitrogen. Use within 8 hours of preparation.
Manufactured by:
Mallinckrodt Inc.
St. Louis, MO 63134 USA
Distributed in Canada by:
tyco Healthcare
Pointe-Claire, QC, Canada H9R 5H8
Estb. Lic. No.: 100689-A
Mallinckrodt
A091V0
R12/2011

TechneScan HDPTechnetium Tc 99m Oxidronate INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||