Technetium Tc99m Generator
TECHNETIUM Tc99m GENERATOR For the Production of Sodium Pertechnetate Tc99m Injection
FULL PRESCRIBING INFORMATION: CONTENTS*
- TECHNETIUM TC99M GENERATOR DESCRIPTION
- CLINICAL PHARMACOLOGY
- TECHNETIUM TC99M GENERATOR INDICATIONS AND USAGE
- TECHNETIUM TC99M GENERATOR CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TECHNETIUM TC99M GENERATOR ADVERSE REACTIONS
- TECHNETIUM TC99M GENERATOR DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PREPARATION
- EXPIRATION DATE
- DISPOSAL
- PRINCIPAL DISPLAY PANEL - 68 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 108 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 135 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 162 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 203 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 230 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 243 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 270 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 338 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 405 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 541 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 676 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 811 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 1,081 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 1,351 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 1,622 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 2,027 mCi Container Label
- PRINCIPAL DISPLAY PANEL - 2,703 mCi Container Label
FULL PRESCRIBING INFORMATION
Diagnostic Radiopharmaceutical
For intravenous use only
TECHNETIUM TC99M GENERATOR DESCRIPTION
The technetium Tc99m generator is prepared with fission-produced molybdenum Mo99 adsorbed on alumina in a lead-shielded column and provides a means for obtaining sterile pyrogen-free solutions of sodium pertechnetate Tc99m injection in sodium chloride. The eluate should be crystal clear. With a pH of 4.5-7.5, hydrochloric acid and/or sodium hydroxide may have been used for Mo99 solution pH adjustment. Over the life of the generator, each elution will provide a yield of > 80% of the theoretical amount of technetium Tc99m available from the molybdenum Mo99 on the generator column.
Each eluate of the generator should not contain more than 0.0056 MBq (0.15 µCi) of molybdenum Mo99 per 37 MBq (1 mCi) of technetium Tc99m per administered dose at the time of administration, and not more than 10 µg of aluminum per mL of the generator eluate, both of which must be determined by the user before administration.
Since the eluate does not contain an antimicrobial agent, it should not be used after twelve hours from the time of generator elution.
PHYSICAL CHARACTERISTICS
Technetium Tc99m decays by an isomeric transition with a physical half-life of 6.02 hours. The principal photon that is useful for detection and imaging studies is listed in Table 1.
| Radiation | Mean %/Disintegration | Mean Energy (keV) |
|---|---|---|
| Gamma-2 | 89.07 | 140.5 |
EXTERNAL RADIATION
The specific gamma ray constant for technetium Tc99m is 1.97 × 10-5 mSv h-1 MBq-1 m2 (0.78 R/hr-mCi) at 1 cm. The first half-value thickness of lead (Pb) for technetium Tc99m is 0.017 cm. A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 2. For example, the use of 0.25 cm of Pb will attenuate the radiation emitted by a factor of about 1,000.
| Shield Thickness (Pb) cm | Coefficient of Attenuation |
|---|---|
| 0.017 | 0.5 |
| 0.08 | 10-1 |
| 0.16 | 10-2 |
| 0.25 | 10-3 |
| 0.33 | 10-4 |
Molybdenum Mo99 decays to technetium Tc99m with a molybdenum Mo99 half-life of 2.75 days. The physical decay characteristics of molybdenum Mo99 are such that only 86.8% of the decaying molybdenum Mo99 nuclei form technetium Tc99m. Generator elutions may be made at any time, but the amount of technetium Tc99m available will depend on the time interval since the last elution. After six hours approximately 47% of maximum technetium Tc99m is available. Ninety-five percent is reached after 24 hours. To correct for physical decay of each radionuclide, the fractions that remain at selected intervals of time are shown in Tables 3a and 3b.
| Days | Percent Remaining | Days | Percent Remaining | Days | Percent Remaining |
|---|---|---|---|---|---|
| -3 | 213.2 | 4 | 36.5 | 11 | 6.3 |
| -2 | 165.6 | 5 | 28.4 | 12 | 4.9 |
| -1 | 128.7 | 6 | 22.0 | 13 | 3.8 |
| 0 |
100.0 | 7 | 17.1 | 14 | 2.9 |
| 1 | 77.7 | 8 | 13.3 | 15 | 2.3 |
| 2 | 60.4 | 9 | 10.3 | 16 | 1.8 |
| 3 | 46.9 | 10 | 8.0 |
| Hours | Percent Remaining | Hours | Percent Remaining |
|---|---|---|---|
| 0 |
100.0 | 7 | 44.7 |
| 1 | 89.1 | 8 | 39.8 |
| 2 | 79.4 | 9 | 35.5 |
| 3 | 70.8 | 10 | 31.6 |
| 4 | 63.1 | 11 | 28.2 |
| 5 | 56.2 | 12 | 25.1 |
| 6 | 50.1 |
CLINICAL PHARMACOLOGY
The pertechnetate ion distributes in the body similarly to the iodide ion, but is not organified when trapped in the thyroid gland. Pertechnetate tends to accumulate in intracranial lesions with excessive neovascularity or an altered blood-brain barrier. It also concentrates in the thyroid gland, salivary glands, gastric mucosa, and choroid plexus. However, in contrast to the iodide ion, the pertechnetate ion is released unchanged from the thyroid gland.
After intravascular administration, the pertechnetate ion remains in the circulatory system for sufficient time to permit blood pool measurement, organ perfusion, and major vessel studies. It gradually equilibrates with the extravascular space. A small fraction is promptly excreted via the kidneys.
Following the administration of sodium pertechnetate Tc99m injection as an eye drop, the drug mixes with tears within the conjunctival space. Within seconds to minutes it leaves the conjunctival space and escapes into the inferior meatus of the nose through the nasolacrimal drainage system. During this process the pertechnetate ion passes through the canaliculi, the lacrimal sac and the nasolacrimal duct. In the event of any anatomical or functional blockage of the drainage system there will be a backflow resulting in tearing (epiphora). Thus, the pertechnetate escapes the conjunctival space in the tears. While the major part of the pertechnetate escapes within a few minutes of normal drainage and tearing, it has been documented that there is some degree of transconjunctival absorption with a fractional turnover rate of 0.015/min. in normal individuals, 0.021/min. in patients without any sac, and 0.027/min. in patients with inflamed conjunctiva due to chronic dacryocystitis. Individual values may vary, but these rates are probably representative and indicate that the maximum possible pertechnetate absorbed will remain below one thousandth of that used in other routine diagnostic procedures.
TECHNETIUM TC99M GENERATOR INDICATIONS AND USAGE
Sodium pertechnetate Tc99m injection is used IN ADULTS as an agent for: brain imaging including cerebral radionuclide angiography; thyroid imaging; salivary gland imaging; placenta localization; blood pool imaging including radionuclide angiography; urinary bladder imaging (direct isotopic cystography) for detection of vesico-ureteral reflux; and nasolacrimal draining system imaging (dacryoscintigraphy).
Sodium pertechnetate Tc99m injection is used IN CHILDREN as an agent for: brain imaging including cerebral radionuclide angiography; thyroid imaging; blood pool imaging including radionuclide angiography; and urinary bladder imaging (direct isotopic cystography) for the detection of vesico-ureteral reflux.
TECHNETIUM TC99M GENERATOR CONTRAINDICATIONS
None known.
WARNINGS
Radiation risks associated with the use of sodium pertechnetate Tc99m injection are greater in children than in adults. In general, the younger the child, the greater the risk owing to greater absorbed radiation doses and longer life expectancy. These greater risks should be taken firmly into account in all benefit-risk assessments involving children.
PRECAUTIONS
General
Technetium Tc99m generators received in advance of the calibration date and time will contain higher amounts of radioactive material. Care should be taken to assure that the generator is properly shielded. As in the use of any radioactive material, care should be taken to minimize radiation exposure to the patient consistent with proper patient management and to insure minimum radiation exposure to occupational workers.
After the termination of the nasolacrimal imaging procedure, blowing the nose and washing the eyes with sterile distilled water or an isotonic sodium chloride solution will further minimize the radiation dose.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential, mutagenic potential, or whether technetium Tc99m may affect fertility in males or females.
Pregnancy Category C
Animal reproductive studies have not been conducted with technetium Tc99m. It is also not known whether technetium Tc99m can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Technetium Tc99m should be given to pregnant women only if the expected benefits to be gained clearly outweigh the potential hazards.
Ideally, examinations using radiopharmaceuticals, especially those elective in nature, of a woman of childbearing capability should be performed during the first few (approximately 10) days following the onset of menses.
Nursing Mothers
Technetium Tc99m is excreted in human milk during lactation, and therefore formula feedings should be substituted for breast feedings.
Pediatric Use
See INDICATIONS AND USAGE, DOSAGE AND ADMINISTRATION . See also description of additional risk under WARNINGS .
TECHNETIUM TC99M GENERATOR ADVERSE REACTIONS
Allergic reactions including anaphylaxis have been reported infrequently following the administration of sodium pertechnetate Tc99m.
TECHNETIUM TC99M GENERATOR DOSAGE AND ADMINISTRATION
Sodium pertechnetate Tc99m injection is usually administered by intravascular injection, but can be given orally. For imaging the urinary bladder and ureters (direct isotopic cystography), the sodium pertechnetate Tc99m injection is instilled aseptically into the bladder via a urethral catheter, following which the catheter is flushed with approximately 200 mL of sterile saline directly into the bladder. The dosage employed varies with each diagnostic procedure. If the oral route is elected, the patient should fast for at least six (6) hours before and two (2) hours after administration. When imaging the nasolacrimal drainage system, instill the sodium pertechnetate Tc99m injection by the use of a device such as a micropipette or similar method which will ensure the accuracy of the dose.
The suggested dose ranges employed for various diagnostic indications in average ADULT patients (70 kg):
| Indication | Megabecquerels (MBq) | Millicuries (mCi) |
|---|---|---|
| Vesico-ureteral imaging | 18.5 - 37.0 | 0.5 - 1 |
| Brain imaging | 370.0 - 740.0 | 10 - 20 |
| Thyroid gland imaging | 37.0 - 370.0 | 1 - 10 |
| Salivary gland imaging | 37.0 - 185.0 | 1 - 5 |
| Placenta localization | 37.0 - 111.0 | 1 - 3 |
| Nasolacrimal drainage system imaging |
3.70 (max.) | 0.100 (max.) |
| Blood pool imaging | 370.0 - 1110.0 | 10 - 30 |
| In PEDIATRIC patients: | |
|---|---|
| NOTE: Up to 1 gram of pharmaceutical grade potassium perchlorate in a suitable base or capsule may be given orally prior to administration of sodium pertechnetate Tc99m injection. When sodium pertechnetate Tc99m injection is used in children for brain or blood pool imaging, administration of potassium perchlorate is especially important to minimize the absorbed radiation dose to the thyroid gland. | |
| Brain imaging | 5.2-10.4 MBq, 140-280 µCi per kilogram body weight. |
| Thyroid gland imaging | 2.2-3.0 MBq, 60-80 µCi per kilogram body weight. |
| Blood pool imaging | 5.2-10.4 MBq, 140-280 µCi per kilogram body weight. A minimum dose of 111.0-185.0 MBq, 3-5 mCi should be employed if radionuclide angiography is performed as part of blood pool imaging procedure. |
| Vesico-ureteral imaging | 18.5-37.0 MBq, 0.5-1.0 mCi |
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
The solution to be administered as the patient dose should be crystal clear and contain no particulate matter.
Since the sodium pertechnetate Tc99m injection eluate does not contain an antimicrobial agent, it should not be used after twelve hours from the time of generator elution.
RADIATION DOSIMETRY
The estimated absorbed radiation doses
| (mGy/1110.0 MBq) | (mGy/111.0 MBq) | ||
|---|---|---|---|
| Tissue | Resting Population | Active Population | |
| NOTE: To convert the radiation dose from milligray (mGy) to rad units, divide the value in milligrays by 10. | |||
| Bladder wall | 15.9 | 25.5 | |
| Gastrointestinal tract: | |||
| Stomach wall | 75.0 | 15.3 | |
| Upper large intestine wall | 20.4 | 36.0 | |
| Lower large intestine wall | 18.3 | 33.0 | |
| Red bone marrow | 5.7 | 5.1 | |
| Testes | 2.7 | 2.7 | |
| Ovaries | 6.6 | 9.0 | |
| Thyroid | 39.0 | 39.0 | |
| Brain | 4.2 | 3.6 | |
| Whole body | 4.2 | 3.3 | |
| Placenta | 0.5 | ||
| Fetus | 0.5 | ||
| Absorbed Radiation Dose | ||
|---|---|---|
| Organ | mGy/3.7 MBq | mrad/100 µCi |
| Eye Lens: | ||
| If lacrimal fluid turnover is 16%/min. | 0.140 | 14.0 |
| If lacrimal fluid turnover is 100%/min. | 0.022 | 2.2 |
| If drainage system is blocked | 4.020 | 402.0 |
| Total Body | 0.011 | 1.1 |
Ovaries |
0.030 | 3.0 |
Testes |
0.009 | 0.9 |
Thyroid |
0.130 | 13.0 |
In PEDIATRIC patients, the maximum radiation doses when a dose of 185.0 MBq, 5 mCi of sodium pertechnetate Tc99m injection is administered to a neonate (3.5 kg) for brain or blood pool imaging with radionuclide angiography are shown in Table 6. When a dose of 37.0 MBq, 1 mCi of sodium pertechnetate Tc99m injection is instilled directly into the urinary bladder for vesico-ureteral imaging in pediatric patients and retained for 30 minutes, the estimated absorbed radiation dose to the bladder wall and ureters is 0.3 mGy, 30 mrad and 0.04-0.05 mGy, 4-5 mrad to the gonads.
| Absorbed Radiation Dose | ||
|---|---|---|
| Tissue | mGy/185.0 MBq | rads/5 mCi |
| Thyroid (without Perchlorate) | 230 | 23.0 |
| Thyroid (with Perchlorate) | 48.5 | 4.85 |
| Large Bowel (with Perchlorate) | 95.5 | 9.55 |
| Testes | 5.1 | 0.51 |
| Ovaries | 11.0 | 1.10 |
| Whole Body | 7.6 | 0.76 |
HOW SUPPLIED
Sodium pertechnetate Tc99m injection is supplied as a molybdenum Mo99 / technetium Tc99m generator in sizes of molybdenum Mo99 from 2.5 GBq up to 100 GBq, 68 mCi up to 2703 mCi, at reference date and time specified on the generator label.
The technetium Tc99m generator contains the following amount of molybdenum Mo99 at the reference time and date stated on the label.
| NDC # | Mo99 (GBq) | Mo99 (mCi) | NDC # | Mo99 (GBq) | Mo99 (mCi) |
|---|---|---|---|---|---|
| 17156-601-51 | 2.5 | 68 | 17156-610-60 | 15 | 405 |
| 17156-602-52 | 4 | 108 | 17156-611-61 | 20 | 541 |
| 17156-603-53 | 5 | 135 | 17156-612-62 | 25 | 676 |
| 17156-604-54 | 6 | 162 | 17156-613-63 | 30 | 811 |
| 17156-605-55 | 7.5 | 203 | 17156-614-64 | 40 | 1,081 |
| 17156-606-56 | 8.5 | 230 | 17156-615-65 | 50 | 1,351 |
| 17156-607-57 | 9 | 243 | 17156-616-66 | 60 | 1,622 |
| 17156-608-58 | 10 | 270 | 17156-617-67 | 75 | 2,027 |
| 17156-609-59 | 12.5 | 338 | 17156-618-68 | 100 | 2,703 |
The technetium Tc99m generator consists of:
- Sterile generator
- Elution pack
The following items are provided in the Elution pack:
- 5 × 30 mL Evacuated vials for collection of the generator eluate
- 5 × 20 mL Saline eluent vials each containing Sodium Chloride Injection 0.9% USP. Not for direct administration.
- 3 Sterile inlet spike protectors - to maintain sterility of the generator system if the saline vial is removed between elutions
- 5 Sterile closed cell foam collection needle protectors - to maintain sterility of the generator system between elutions
- 5 Spare sterile needles - to enable the user to replace the collection needle
- 6 Courtesy labels - to record the activity, volume and time of elution
- 1 Package insert
Additional quantities of these components are supplied at the customer's request to allow further elution of the generator. Additional components will be supplied as either one of the following two packs:
Saline vials pack
The saline vials pack is available in 5 mL, 10 mL or 20 mL volume to allow the generator eluate to be collected at varying radioactive concentrations. Each pack contains 20 vials of Sodium Chloride Injection 0.9% USP, not for direct administration, packed in an outer carton.
Evacuated vials pack
Each evacuated vials pack contains the following components for the elution of the generator packed in an outer carton.
- 10 × 30 mL Evacuated vials for collection of the generator eluate
- 6 Sterile inlet spike protectors - to maintain sterility of the generator system if the saline vial is removed between elutions
- 10 Sterile closed cell foam collection needle protectors - to maintain sterility of the generator system between elutions
- 10 Spare sterile needles - to enable the user to replace the collection needle
- 12 Courtesy labels - to record the activity, volume and time of elution
Storage
Store the generator and the eluate, sodium pertechnetate Tc99m Injection, below 25°C (77°F). Do not freeze.
Store the saline eluent vial below 25°C (77°F). Do not freeze.
Storage should be in accordance with local regulations for radioactive materials.
PREPARATION
This radiopharmaceutical may be received, used and administered only by authorized persons in designated clinical settings. Their receipt, storage, use, transfer and disposal are subject to the regulations and/or appropriate licenses of the regulatory authorities (see DISPOSAL ).
The administration of radiopharmaceuticals creates risks for other persons from external radiation or contamination from spills of urine, vomiting, etc. Take appropriate radiation protection precautions in accordance with national regulations.
Instructions for elution of the technetium Tc99m generator
Elution instructions
The facilities used for elutions should comply with the appropriate regulations for safe radiological handling. Strict aseptic techniques should be used during the elution of the generator to ensure sterility of the generator eluate. To avoid unsatisfactory performance it is important to adhere to the following sequence of elution steps.
FIRST ELUTION
-
(1) Remove the generator and accompanying accessories from their packaging. Place the generator on a flat, level surface, in a suitably authorized and shielded location. Do not remove the spike and needle protectors until you are ready to carry out the first elution. -
(2) Select a saline vial containing the required volume of saline. -
(3) Remove the flip-top from the saline vial and swab the saline vial closure using a bactericidal swab and allow to dry. -
(4) Remove the spike protector. -
(5) Place the saline vial onto the spike, ensuring that it is fully pushed to the bottom of the inlet well. Partial rotation will assist the positioning of the vial. -
(6) Select an evacuated collection vial, remove the flip top cap, and swab the collection vial closure using a bactericidal swab and allow to dry. Prior to placing the collection vial inside the collection vial shield, remove the shield lid and ensure that the vial contact surfaces of the shield have been swabbed using the bactericidal swab provided. Replace the collection vial shield screw-locked cap. The collection shield push- fit top is not required until the elution has been completed. -
(7) Remove the collection point protector by turning it counter-clockwise. Ensure that the luer type filter attached to the collection point protector is also removed. Retain the collection point protector for use when returning the generator. Immediately fit a collection needle provided in the accessory pack. Do not remove the collection needle sheath until you are ready to place the collection vial on the needle. -
(8) Remove the collection needle sheath and place the collection vial shield on to the collection needle, aligning the side location into its guide, and the window to the front. Push down to ensure that the vial is fully located on the collection needle. -
(9) Allow at least 3 minutes for the elution to complete. Elution is complete when all vigorous bubbling has ceased inside the collection vial. Do not remove either the saline vial or collection vial until after elution is complete. -
(10) Slowly remove the collection vial shield to prevent damage to the collection needle and replace the push-fit top for added radiation protection. -
(11) Cover with a new collection needle protector from the accessory pack to preserve sterility. -
(12) To preserve sterility leave the empty saline vial in place until the next elution.
SUBSEQUENT ELUTIONS
Using a new sanitized saline vial of the required volume repeat steps 5–12.
If the collection needle needs to be changed, remove the damaged needle and swab the collection well to ensure sterility is maintained and fit a new needle. Place a collection needle protector over the new needle.
Following expiry, a spare spike protector and the retained collection point protector should be used to cover the spike and collection point respectively.
ELUTION VOLUME AND YIELD OF TECHNETIUM Tc99m
Due to the elution characteristics of the different column designs, the recommended minimum elution volume for lead shielded generators is 5 mL (2.5 to 30 GBq Mo99). For depleted uranium shielded generators (40 to 100 GBq Mo99) the recommended minimum elution volume is 10 mL. If 5 mL elutions are used a higher radioactive concentration will be obtained, but a small yield reduction is likely.
The technetium Tc99m generator is calibrated in terms of the amount of molybdenum Mo99 loaded on the column. The available technetium Tc99m at any time depends on the time before or after reference (due to the decay of molybdenum Mo99, the time elapsed since the previous elution (due to "growth" of technetium Tc99m) and on the decay characteristics of molybdenum Mo99 (86.8% of all decay yields technetium Tc99m). The percentages listed in Tables 7 and 8 may be used to calculate the available technetium Tc99m activity using the following method.
First, multiply the stated reference activity by the appropriate factor from Table 7 (which allows for decay of molybdenum Mo99). Then multiply the product by the appropriate percentage from Table 8 (which allows for the growth of technetium Tc99m and for decay characteristics of molybdenum Mo99).
The actual yield of technetium Tc99m will vary slightly due to variation in elution efficiency from generator to generator. It will typically not be less than 90% of the available technetium Tc99m activity.
| Days from generator reference time | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (hrs) | -10 | -9 | -8 | -7 | -6 | -5 | -4 | -3 | -2 | -1 | 0 | 1 |
| 2.00 | 13.8123 | 10.7349 | 8.3432 | 6.4844 | 5.0397 | 3.9169 | 3.0442 | 2.3660 | 1.8388 | 1.4291 | 1.1107 | 0.8633 |
| 4.00 | 13.5252 | 10.5118 | 8.1698 | 6.3496 | 4.9349 | 3.8354 | 2.9809 | 2.3168 | 1.8006 | 1.3994 | 1.0876 | 0.8453 |
| 6.00 | 13.2441 | 10.2933 | 8.0000 | 6.2176 | 4.8324 | 3.7557 | 2.9190 | 2.2686 | 1.7632 | 1.3704 | 1.0650 | 0.8278 |
| 8.00 | 12.9688 | 10.0794 | 7.8337 | 6.0884 | 4.7319 | 3.6777 | 2.8583 | 2.2215 | 1.7265 | 1.3419 | 1.0429 | 0.8105 |
| 10.00 | 12.6992 | 9.8699 | 7.6709 | 5.9618 | 4.6336 | 3.6012 | 2.7989 | 2.1753 | 1.6906 | 1.3140 | 1.0212 | 0.7937 |
| 12.00 | 12.4353 | 9.6647 | 7.5114 | 5.8379 | 4.5373 | 3.5264 | 2.7407 | 2.1301 | 1.6555 | 1.2867 | 1.0000 | 0.7772 |
| 14.00 | 12.1768 | 9.4638 | 7.3553 | 5.7166 | 4.4429 | 3.4531 | 2.6837 | 2.0858 | 1.6211 | 1.2599 | 0.9792 | 0.7610 |
| 16.00 | 11.9237 | 9.2671 | 7.2024 | 5.5978 | 4.3506 | 3.3813 | 2.6280 | 2.0425 | 1.5874 | 1.2337 | 0.9589 | 0.7452 |
| 18.00 | 11.6758 | 9.0745 | 7.0527 | 5.4814 | 4.2602 | 3.3110 | 2.5733 | 2.0000 | 1.5544 | 1.2081 | 0.9389 | 0.7297 |
| 20.00 | 11.4332 | 8.8859 | 6.9061 | 5.3675 | 4.1716 | 3.2422 | 2.5198 | 1.9584 | 1.5221 | 1.1830 | 0.9194 | 0.7146 |
| 22.00 | 11.1955 | 8.7012 | 6.7626 | 5.2559 | 4.0849 | 3.1748 | 2.4675 | 1.9177 | 1.4905 | 1.1584 | 0.9003 | 0.6997 |
| 24.00 | 10.9628 | 8.5203 | 6.6220 | 5.1467 | 4.0000 | 3.1088 | 2.4162 | 1.8779 | 1.4595 | 1.1343 | 0.8816 | 0.6852 |
| Days from generator reference time | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (hrs) | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 2.00 | 0.6709 | 0.5215 | 0.4053 | 0.3150 | 0.2448 | 0.1903 | 0.1479 | 0.1149 | 0.0893 | 0.0694 | 0.0540 | 0.0419 | 0.0326 |
| 4.00 | 0.6570 | 0.5106 | 0.3969 | 0.3084 | 0.2397 | 0.1863 | 0.1448 | 0.1125 | 0.0875 | 0.0680 | 0.0528 | 0.0411 | 0.0319 |
| 6.00 | 0.6433 | 0.5000 | 0.3886 | 0.3020 | 0.2347 | 0.1824 | 0.1418 | 0.1102 | 0.0856 | 0.0666 | 0.0517 | 0.0402 | 0.0313 |
| 8.00 | 0.6300 | 0.4896 | 0.3805 | 0.2957 | 0.2299 | 0.1786 | 0.1388 | 0.1079 | 0.0839 | 0.0652 | 0.0507 | 0.0394 | 0.0306 |
| 10.00 | 0.6169 | 0.4794 | 0.3726 | 0.2896 | 0.2251 | 0.1749 | 0.1360 | 0.1057 | 0.0821 | 0.0638 | 0.0496 | 0.0386 | 0.0300 |
| 12.00 | 0.6040 | 0.4695 | 0.3649 | 0.2836 | 0.2204 | 0.1713 | 0.1331 | 0.1035 | 0.0804 | 0.0625 | 0.0486 | 0.0378 | 0.0293 |
| 14.00 | 0.5915 | 0.4597 | 0.3573 | 0.2777 | 0.2158 | 0.1677 | 0.1304 | 0.1013 | 0.0787 | 0.0612 | 0.0476 | 0.0370 | 0.0287 |
| 16.00 | 0.5792 | 0.4502 | 0.3499 | 0.2719 | 0.2113 | 0.1642 | 0.1277 | 0.0992 | 0.0771 | 0.0599 | 0.0466 | 0.0362 | 0.0281 |
| 18.00 | 0.5672 | 0.4408 | 0.3426 | 0.2663 | 0.2069 | 0.1608 | 0.1250 | 0.0972 | 0.0755 | 0.0587 | 0.0456 | 0.0354 | 0.0275 |
| 20.00 | 0.5554 | 0.4316 | 0.3355 | 0.2607 | 0.2026 | 0.1575 | 0.1224 | 0.0951 | 0.0739 | 0.0575 | 0.0447 | 0.0347 | 0.0270 |
| 22.00 | 0.5438 | 0.4227 | 0.3285 | 0.2553 | 0.1984 | 0.1542 | 0.1199 | 0.0932 | 0.0724 | 0.0563 | 0.0437 | 0.0340 | 0.0264 |
| 24.00 | 0.5325 | 0.4139 | 0.3217 | 0.2500 | 0.1943 | 0.1510 | 0.1174 | 0.0912 | 0.0709 | 0.0551 | 0.0428 | 0.0333 | 0.0259 |
| Hours | Factor | Hours | Factor | Hours | Factor | Hours | Factor | Hours | Factor | Hours | Factor |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.094 | 9 | 0.579 | 17 | 0.788 | 25 | 0.879 | 33 | 0.918 | 41 | 0.936 |
| 2 | 0.179 | 10 | 0.615 | 18 | 0.804 | 26 | 0.884 | 34 | 0.921 | 42 | 0.937 |
| 3 | 0.256 | 11 | 0.648 | 19 | 0.818 | 27 | 0.892 | 35 | 0.924 | 43 | 0.938 |
| 4 | 0.324 | 12 | 0.678 | 20 | 0.831 | 28 | 0.898 | 36 | 0.926 | 44 | 0.940 |
| 5 | 0.386 | 13 | 0.705 | 21 | 0.843 | 29 | 0.903 | 37 | 0.929 | 45 | 0.941 |
| 6 | 0.442 | 14 | 0.729 | 22 | 0.853 | 30 | 0.907 | 38 | 0.930 | 46 | 0.941 |
| 7 | 0.492 | 15 | 0.751 | 23 | 0.863 | 31 | 0.911 | 39 | 0.932 | 47 | 0.941 |
| 8 | 0.538 | 16 | 0.771 | 24 | 0.871 | 32 | 0.915 | 40 | 0.934 | 48 | 0.942 |
TECHNETIUM Tc99m ASSAY PROCEDURE
The sodium pertechnetate Tc99m injection eluate may be assayed using an ionization chamber dose calibrator. The manufacturer's instructions for operation of the dose calibrator should be followed for measurement of technetium Tc99m and molybdenum Mo99 activity in the generator eluate. The molybdenum Mo99 / technetium Tc99m ratio should be determined at the time of elution prior to administration, and from that ratio, the expiration time (up to 12 hours) of the eluate mathematically determined. Each eluate should meet or exceed the purity requirements of the current United States Pharmacopeia; that is, not more than 0.0056 MBq (0.15 µCi) of molybdenum Mo99 per 37 MBq (1 mCi) of technetium Tc99m per administered dose at the time of administration.
RADIOMETRIC MOLYBDENUM TEST PROCEDURE
This method is based on the fact that most technetium Tc99m radiation can be readily shielded and only the more energetic gamma rays from molybdenum Mo99 (739KeV and 778KeV) are counted in the 550-850KeV energy range. The entire sodium pertechnetate Tc99m injection eluate may be assayed for molybdenum Mo99 activity as follows:
- A cesium Cs137 reference source which has the same geometry as the generator eluate must be used to standardize the well counter.
- Determine the background after setting the window to the 550-850KeV energy range.
- Count the technetium Tc99m eluate in its lead shield (thereby shielding out technetium Tc99m) by placing over the well or probe.
- Count the cesium Cs137 reference source in the same shield geometry for the same time period.
- Compute molybdenum Mo99 activity in the sodium pertechnetate Tc99m injection eluate as follows:
| μCi molybdenum = | μCi simulated Mo99 × net cpm Eluate |
| Mo99 (total) | net cpm simulated Mo99 reference source |
Divide this number by the mCi of technetium Tc99m. This result (µCi Mo99/mCi Tc99m) can be converted to MBq Mo99/MBq Tc99m by multiplying by 10-3. The U.S. Pharmacopeia and the U.S. Nuclear Regulatory Commission or equivalent Agreement State regulations specify a limit of 0.0056 MBq of molybdenum Mo99 per 37 MBq of technetium Tc99m (0.15 µCi of Mo99 / mCi of Tc99m) at the time of administration to each patient.
MOLYBDENUM Mo99 BREAKTHROUGH TEST
- Determine the amount of technetium Tc99m eluted (MBq, mCi).
- Place the sodium pertechnetate Tc99m injection eluate in a lead container. Place lid on container and put the entire container in the chamber.
- Record the amount of molybdenum Mo99 (MBq, mCi) on the most sensitive scale.
- Divide the MBq, mCi molybdenum Mo99 by the MBq, mCi technetium Tc99m. Correct for decay and shielding effect, if necessary.
The molybdenum Mo99/technetium Tc99m ratio should be determined at the time of elution prior to administration, and from that ratio, the expiration time (up to twelve hours) of the eluate mathematically determined. Each sodium pertechnetate Tc99m injection eluate should meet or exceed purity requirement of the current official United States Pharmacopeia.
COLORIMETRIC ALUMINIUM TEST PROCEDURE
Obtain an aluminium ion indicator kit and determine the aluminium ion concentration of the sodium pertechnetate Tc99m injection eluate per the manufacturer's instructions. The concentration must not exceed 10 µg/mL of eluate.
DISPOSAL
All components shipped with the technetium Tc99m generator should be monitored for contamination prior to disposing into routine trash systems. The technetium Tc99m should not be disposed of into routine trash systems. The generator should be disposed through a USNRC or Agreement State licensed disposal agency or by a method approved by the appropriate regulatory authority. Spent generators may be returned; full return instructions of generators to GE Healthcare are provided separately and are available upon request.
EXPIRATION DATE
The expiry date for the generator is 24 days from the date of manufacture. The reference and expiry dates are stated on the generator label. The technetium Tc99m generator should not be used after expiration date.
For multidose use, the sodium pertechnetate Tc99m injection eluate should be used within twelve (12) hours of the generator elution time. If the eluate is used to reconstitute a kit, the radio-labeled kit should not be used after twelve (12) hours from the time of generator elution or six (6) hours after reconstitution of the kit, whichever is earlier.
The shelf-life for the sodium chloride eluent is 3 years.
DISPOSAL
Expired generators containing lead shielding (2.5 to 30 GBq Mo99) could normally be disposed of by the user as radioactive waste in accordance with the conditions specified by the local regulatory authority. If local regulations for disposal require that the generator be dismantled, please contact GE Healthcare. Arrangements may be made for the return of lead shielded generators to GE Healthcare if required.
Generators containing depleted uranium and tungsten shielding (40 to 100 GBq Mo99) must be returned to GE Healthcare after expiry. Full return instructions describing how to return generators to GE Healthcare are provided separately. Users are reminded that all packaging, documentation and methods of transportation used must be in compliance with international transport regulations and all local regulations and codes of practice that relate to such matters.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the US NRC or an Agreement State.
GE Healthcare, Medi-Physics, Inc.
Arlington Heights, IL 60004 U.S.A.
Manufactured by:
GE Healthcare Ltd., HP7 9LL, UK
Customer Service: 1-800-292-8514
Professional Services: 1-800-654-0118
GE and the GE Monogram are trademarks of General Electric Company.
© 2013 General Electric Company - All rights reserved.
43-3500H
Revised November 2013
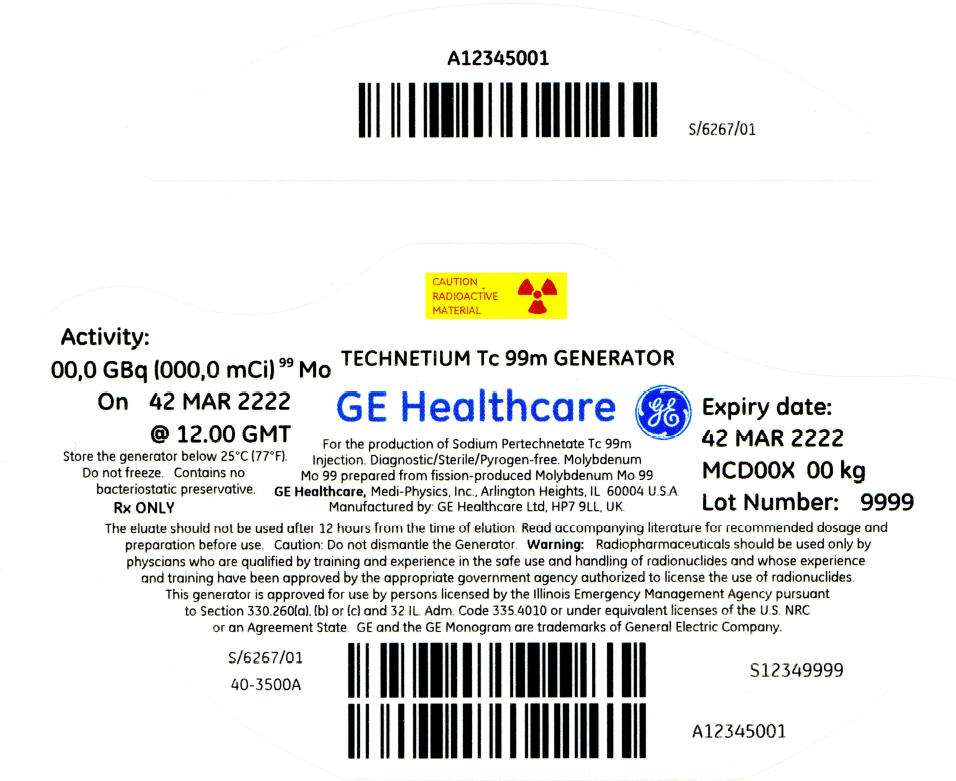
PRINCIPAL DISPLAY PANEL - 68 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
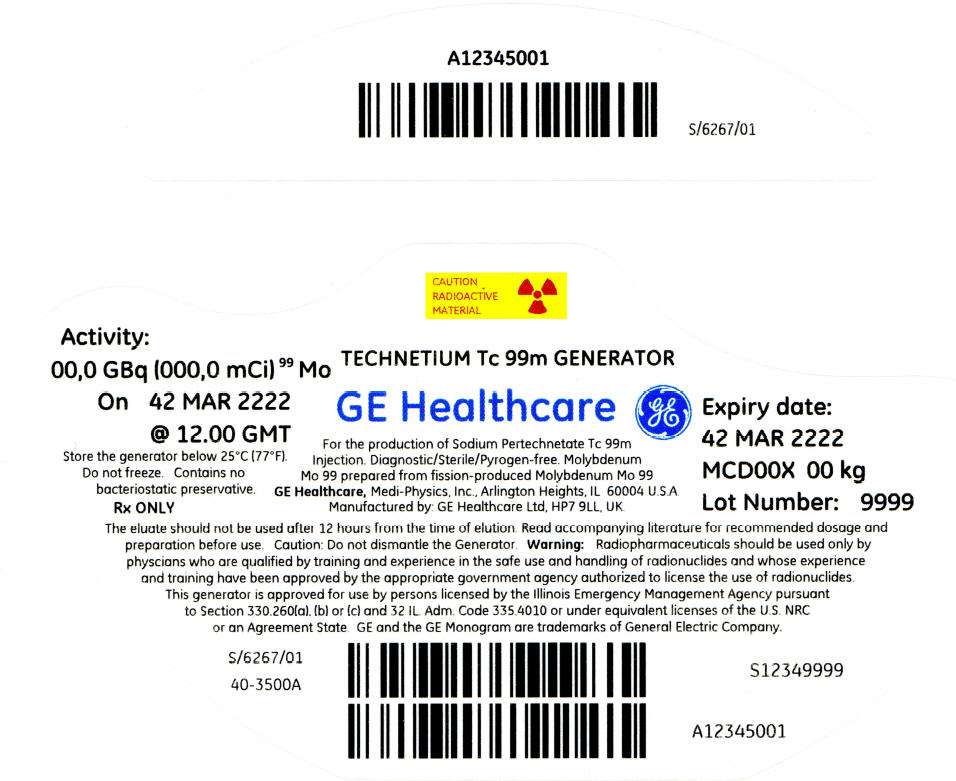
PRINCIPAL DISPLAY PANEL - 108 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
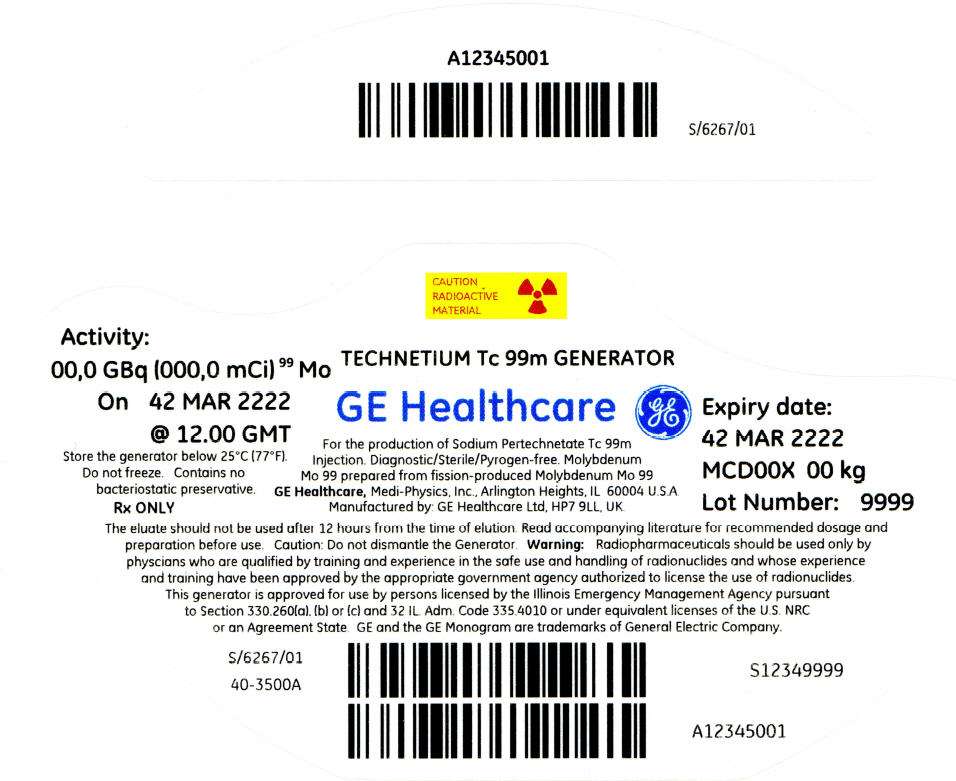
PRINCIPAL DISPLAY PANEL - 135 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
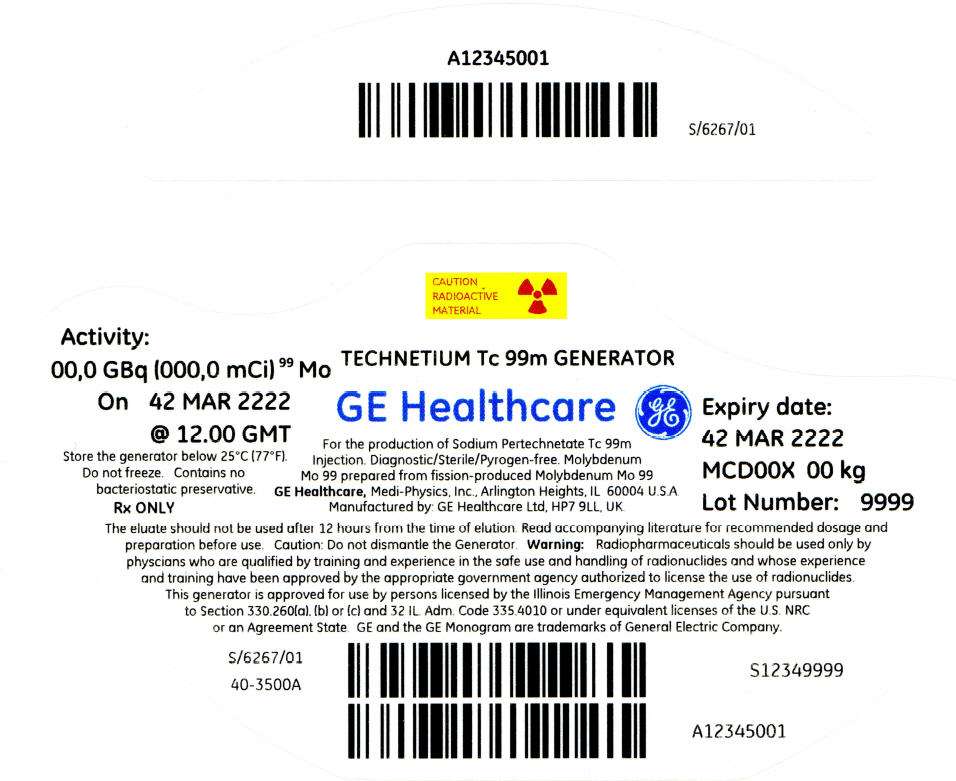
PRINCIPAL DISPLAY PANEL - 162 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.

PRINCIPAL DISPLAY PANEL - 203 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
PRINCIPAL DISPLAY PANEL - 230 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
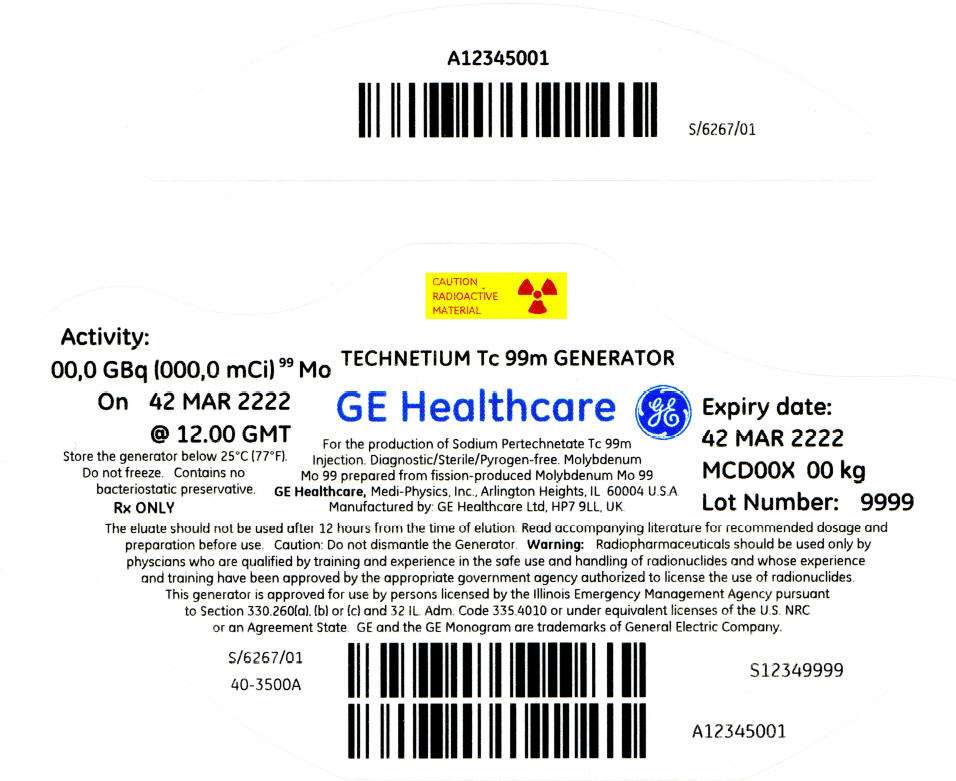
PRINCIPAL DISPLAY PANEL - 243 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
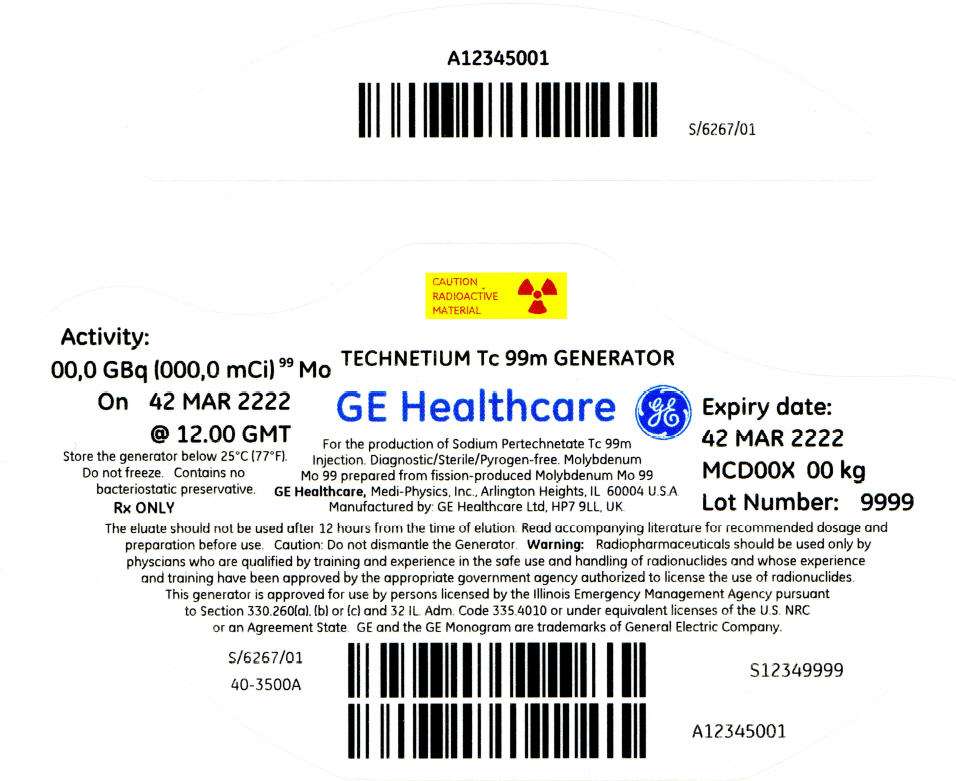
PRINCIPAL DISPLAY PANEL - 270 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
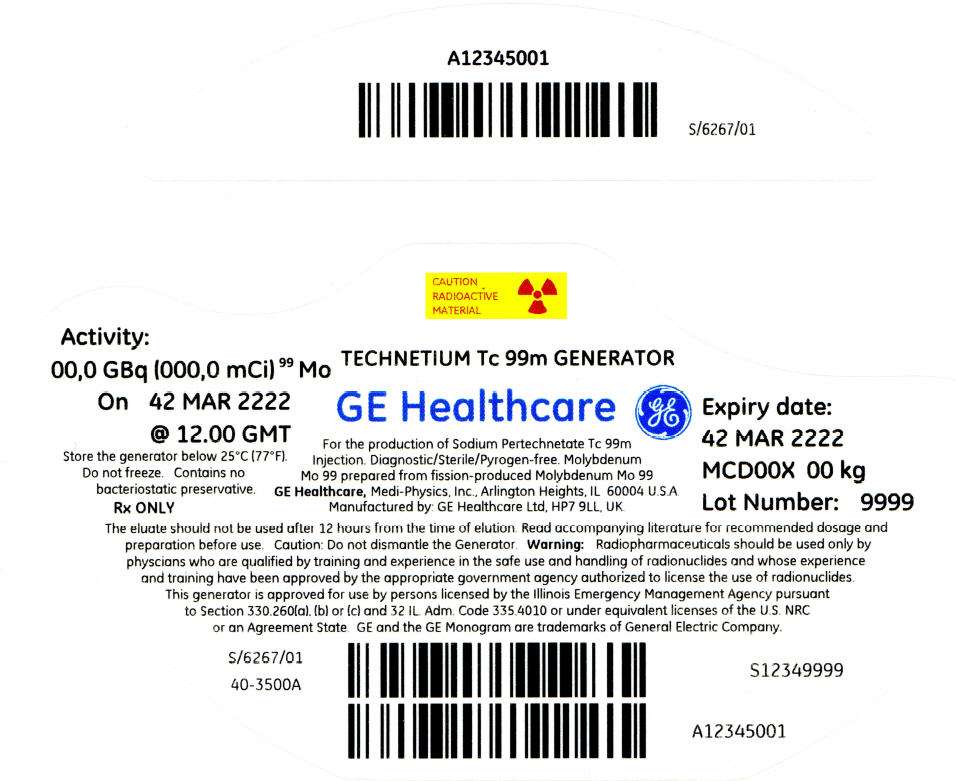
PRINCIPAL DISPLAY PANEL - 338 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
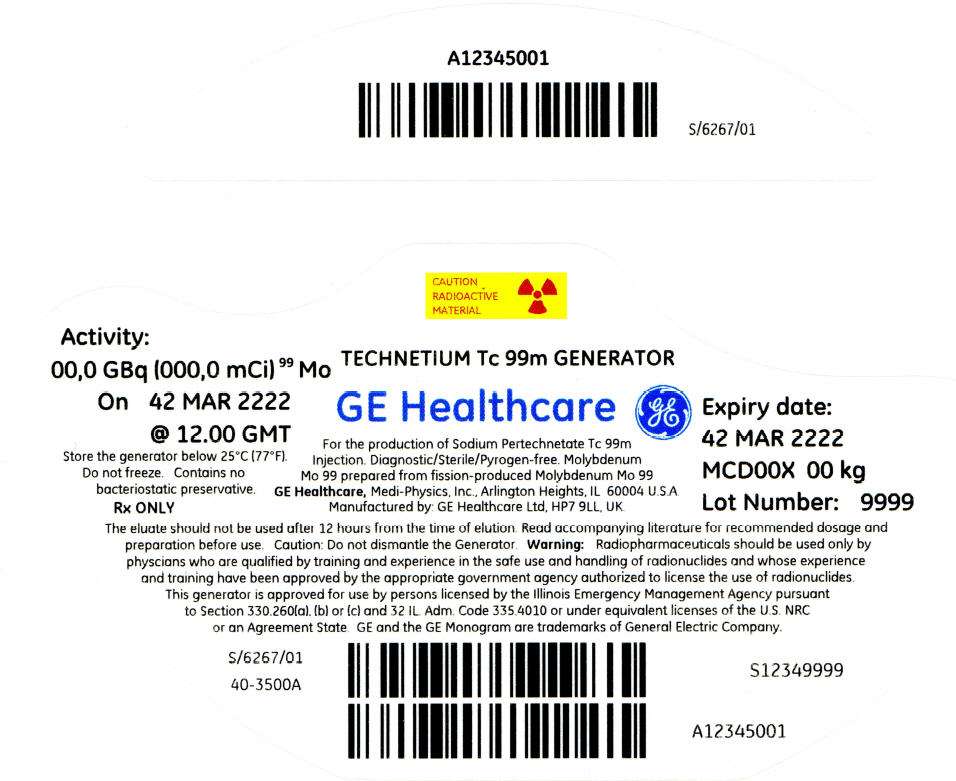
PRINCIPAL DISPLAY PANEL - 405 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
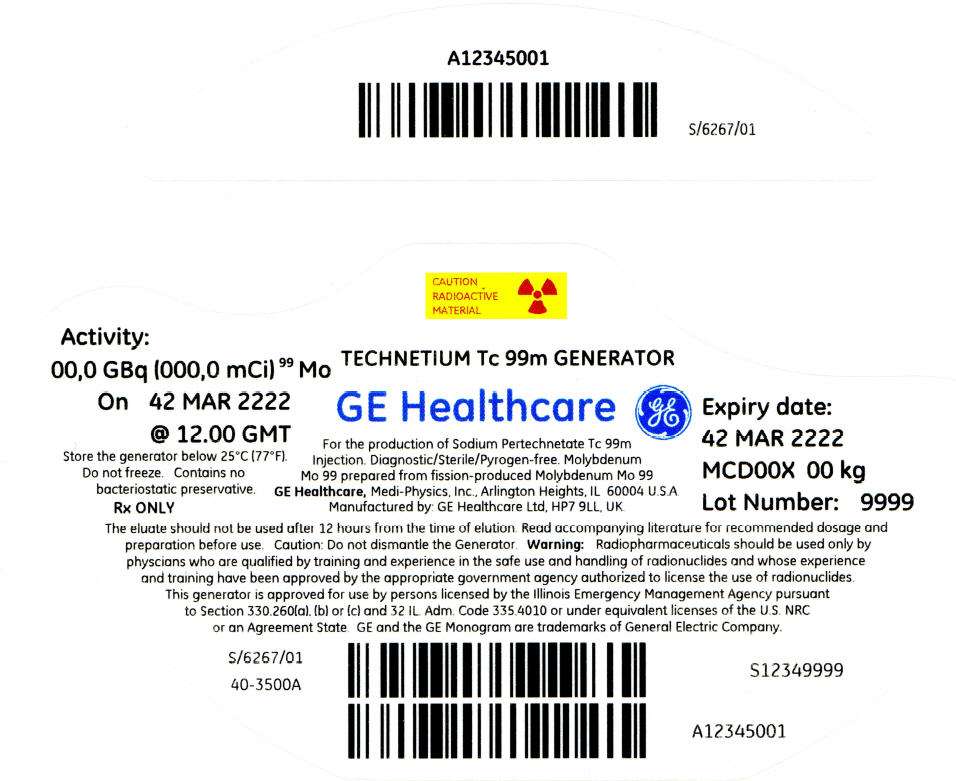
PRINCIPAL DISPLAY PANEL - 541 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
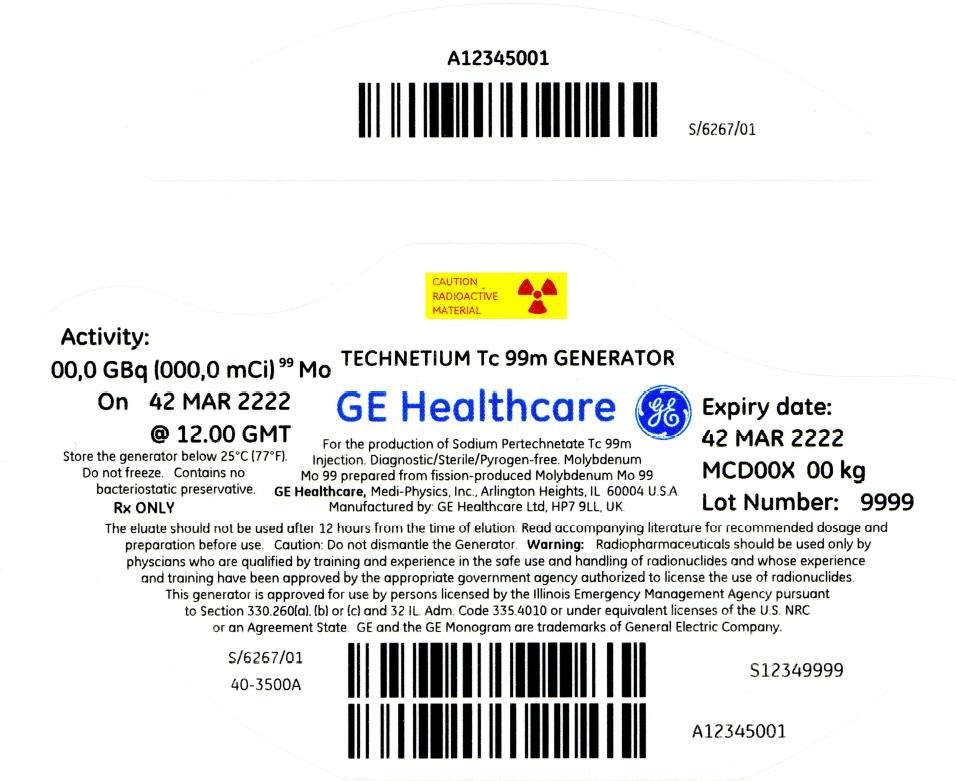
PRINCIPAL DISPLAY PANEL - 676 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
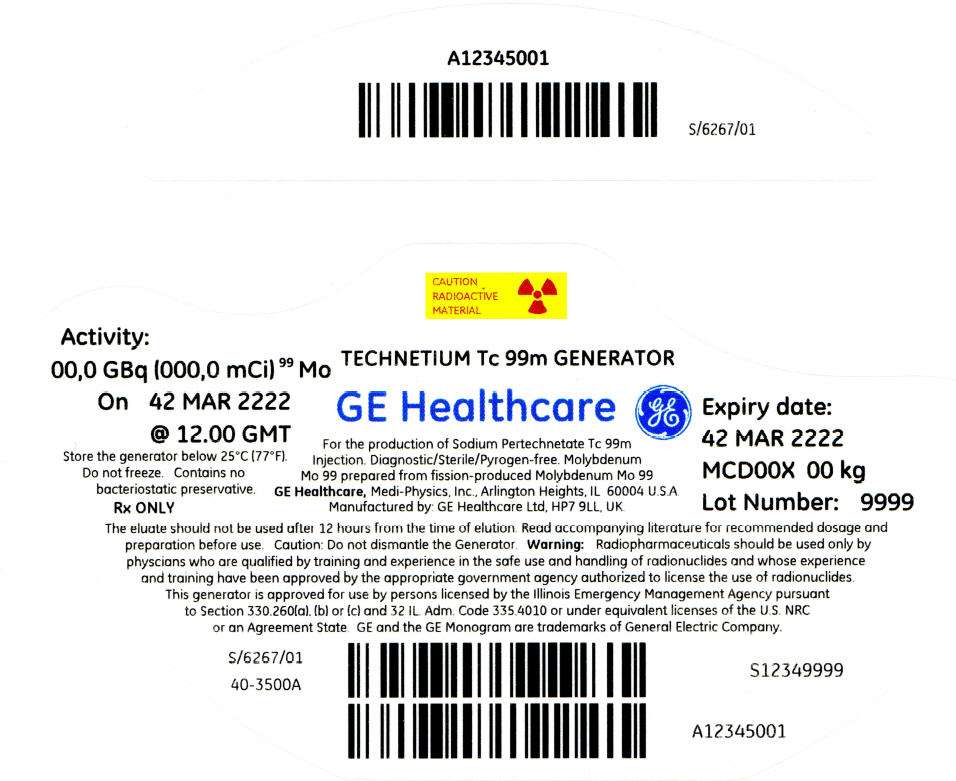
PRINCIPAL DISPLAY PANEL - 811 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
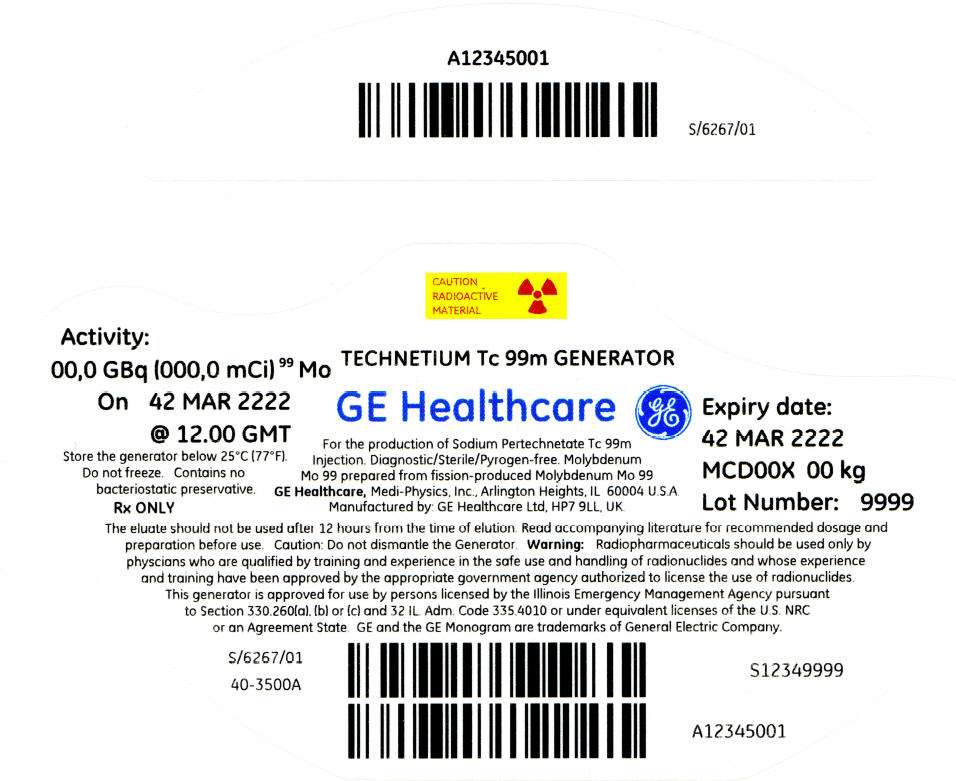
PRINCIPAL DISPLAY PANEL - 1,081 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
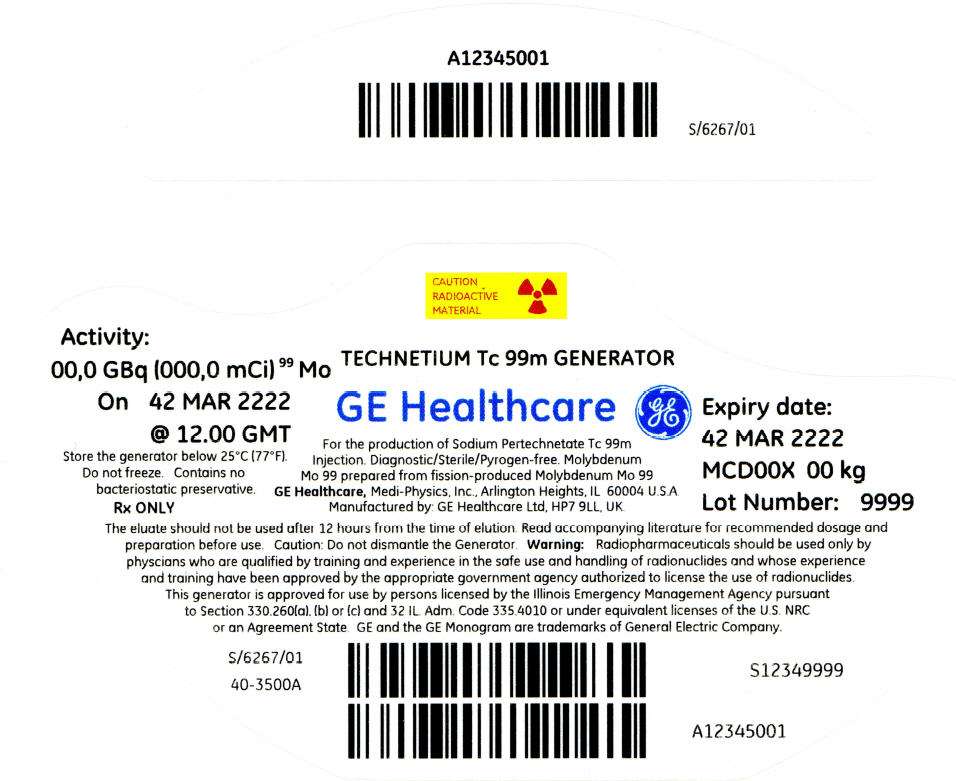
PRINCIPAL DISPLAY PANEL - 1,351 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
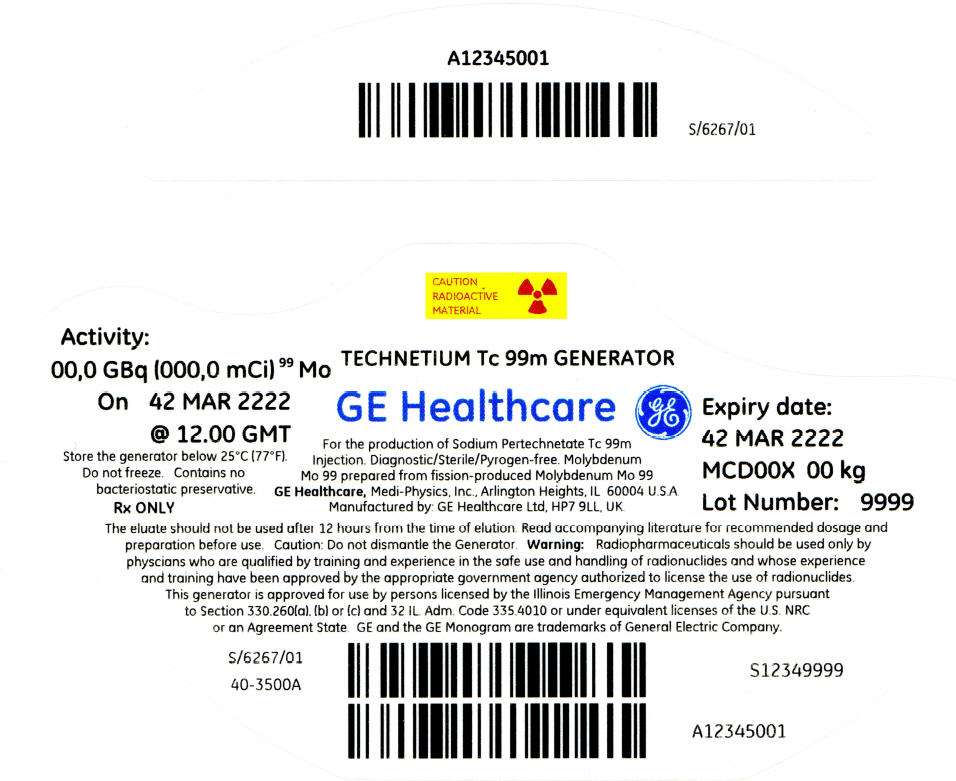
PRINCIPAL DISPLAY PANEL - 1,622 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.
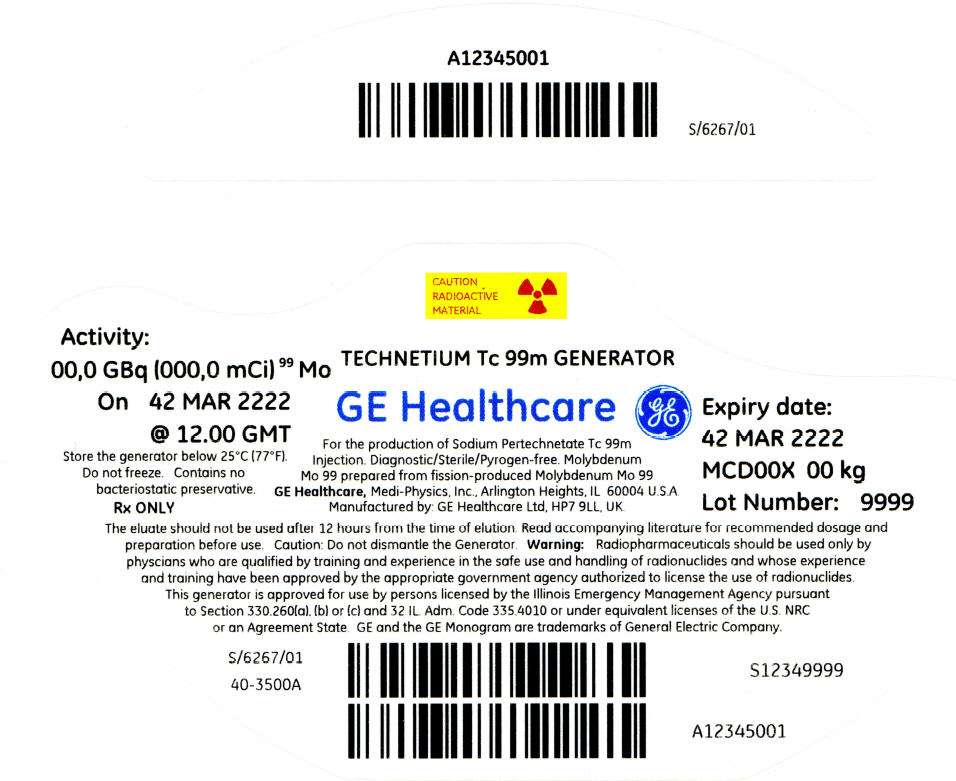
PRINCIPAL DISPLAY PANEL - 2,027 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.

PRINCIPAL DISPLAY PANEL - 2,703 mCi Container Label
CAUTION
RADIOACTIVE
MATERIAL
Activity:
00,0 GBq (000,0 mCi)99 Mo
On 42 MAR 2222
@ 12.00 GMT
Store the generator below 25°C (77°F).
Do not freeze. Contains no
bacteriostatic preservative.
Rx ONLY
TECHNETIUM Tc 99m GENERATOR
GE Healthcare
For the production of Sodium Pertechnetate Tc 99m
Injection. Diagnostic/Sterile/Pyrogen-free. Molybdenum
Mo 99 prepared from fission-produced Molybdenum Mo 99
GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
Manufactured by: GE Healthcare Ltd, HP7 9LL, UK.
Expiry date:
42 MAR 2222
MCD00X 00 kg
Lot Number: 9999
The eluate should not be used after 12 hours from the time of elution. Read accompanying literature for recommended dosage and
preparation before use. Caution: Do not dismantle the Generator. Warning: Radiopharmaceuticals should be used only by
physcians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience
and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
This generator is approved for use by persons licensed by the Illinois Emergency Management Agency pursuant
to Section 330.260(a), (b) or (c) and 32 IL. Adm. Code 335.4010 or under equivalent licenses of the U.S. NRC
or an Agreement State. GE and the GE Monogram are trademarks of General Electric Company.

Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||
Technetium Tc99m GeneratorTECHNETIUM TC-99M SODIUM PERTECHNETATE KIT
| ||||||||||||||||||||||||||||||||||||||||