Tekamlo
Novartis Pharmaceuticals Corporation
HIGHLIGHTS OF PRESCRIBING INFORMATION RECENT MAJOR CHANGESWarnings and Precautions (5.4) 11/2013BOXED WARNING WARNING: FETAL TOXICITY See full prescribing information for complete boxed warning. When pregnancy is detected, discontinue Tekamlo as soon as possible. (5.1) Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. (5.1) INDICATIONS AND USAGETekamlo is a combination of aliskiren, a renin inhibitor, and amlodipine, a dihydropyridine calcium channel blocker, indicated for the treatment of hypertension, to lower blood pressure: As initial therapy in patients likely to need multiple drugs to achieve their blood pressure goals. (1) In patients not adequately controlled with monotherapy. (1) As a substitute for its titrated components. (1) Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions.DOSAGE AND ADMINISTRATION Add-on therapy or initial therapy: Initiate with 150 mg/5 mg. Titrate as needed up to a maximum of 300 mg/10 mg. (2.1, 2.2) The blood pressure lowering effect is largely attained within 1-2 weeks. (2.1) Replacement therapy: may substitute for titrated components. (2.3) Administer one tablet daily with a routine pattern with regard to meals. (2.5) DOSAGE FORMS AND STRENGTHSTablets (aliskiren/amlodipine): 150 mg/5 mg, 150 mg/10 mg, 300 mg/5 mg, 300 mg/10 mg. (3)CONTRAINDICATIONSDo not use with angiotensin receptor blockers (ARBs) or ACE inhibitors (ACEIs) in patients with diabetes (4)Known hypersensitivity to any component (4)WARNINGS AND PRECAUTIONS Avoid concomitant use with ARBs or ACEI in patients with renal impairment (GFR
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
- When pregnancy is detected, discontinue Tekamlo as soon as possible. (5.1)
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. (5.1)
Tekamlo is indicated for the treatment of hypertension, alone or with other antihypertensive agents, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including amlodipine. There are no controlled trials demonstrating risk reduction with Tekamlo.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Data from the high-dose multifactorial study [see Clinical Studies (14)] provide estimates of the probability of reaching a target blood pressure with Tekamlo compared to aliskiren or amlodipine monotherapy. The figures below provide estimates of the likelihood of achieving systolic or diastolic blood pressure control with Tekamlo 300 mg/10 mg, based upon baseline systolic or diastolic blood pressure. The curve of each treatment group was estimated by logistic regression modeling. The estimated likelihood at the right tail of each curve is less reliable because of a small number of subjects with high baseline blood pressures.
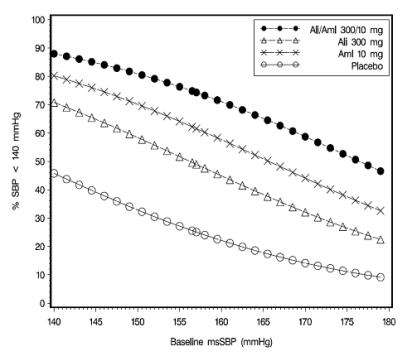
The figures above provide an approximation of the likelihood of reaching a targeted blood pressure goal (e.g. SBP<140 mmHg or <130 mmHg) for the high dose groups evaluated in the study. At all levels of baseline blood pressure, the probability of achieving any given diastolic or systolic goal is greater with the combination than for either monotherapy. For example, the mean baseline SBP/DBP for patients participating in this multifactorial study was 157/100 mmHg. A patient with a baseline blood pressure of 157/100 mmHg has about a 49% likelihood of achieving a goal of <140 mmHg (systolic) and 50% likelihood of achieving <90 mmHg (diastolic) on aliskiren alone, and the likelihood of achieving these goals on amlodipine alone is about 62% (systolic) and 69% (diastolic). The likelihood of achieving these goals on Tekamlo rises to about 74% (systolic) and 83% (diastolic). The likelihood of achieving these goals on placebo is about 25% (systolic) and 27% (diastolic) [ see Dosage and Administration (2) and Clinical Studies (14) ].
2.1 General Considerations
The recommended initial once-daily dose of Tekamlo is 150 mg/5 mg. Titrate as needed to a maximum of 300 mg/10 mg.
The blood pressure lowering effects are largely attained within 1-2 weeks. If blood pressure remains uncontrolled after 2 to 4 weeks of therapy, titrate the dose to a maximum of Tekamlo 300 mg/10 mg once daily.
2.2 Add-on Therapy
Use Tekamlo for patients not adequately controlled with aliskiren alone or amlodipine besylate (or another dihydropyridine calcium channel blocker) alone.
Switch a patient who experiences dose-limiting adverse reactions on either component alone to Tekamlo containing a lower dose of that component in combination with the other to achieve similar blood pressure reductions.
2.3 Replacement Therapy
Switch patients receiving aliskiren and amlodipine besylate from separate tablets to a single tablet of Tekamlo containing the same component doses. When substituting for individual components, increase the dose of one or both of the components if blood pressure control has not been satisfactory.
2.4 Use with Other Antihypertensive Drugs
Tekamlo may be administered with some other antihypertensive agents. In diabetics, do not use in combination with angiotensin receptor blockers (ARBs) or angiotensin converting enzyme inhibitors (ACEIs) [see Contraindications (4)]. Concomitant use of aliskiren with an ARB or ACEI is not recommended in patients with GFR <60 ml/min [see Warnings and Precautions (5.2)]. It is not known whether Tekamlo decreases blood pressure further when added to maximum dosages of ACE inhibitors and beta blockers [see Clinical Studies (14)].
2.5 Relationship to Meals
Advise patients to establish a routine pattern for taking Tekamlo with regard to meals. High-fat meals decrease absorption substantially [ see Clinical Pharmacology (12.3) ] .
- 150 mg aliskiren/5 mg amlodipine tablets: Non-scored light yellow, ovaloid convex shaped film-coated tablet with a beveled edge with debossing “T2” on one side and “NVR” on the reverse side of the tablet.
- 150 mg aliskiren/10 mg amlodipine tablets: Non-scored yellow, ovaloid convex shaped film-coated tablet with a beveled edge with debossing “T7” on one side and “NVR” on the reverse side of the tablet.
- 300 mg aliskiren/5 mg amlodipine tablets: Non-scored dark yellow, ovaloid convex shaped film-coated tablet with a beveled edge with debossing “T11” on one side and “NVR” on the reverse side of the tablet.
- 300 mg aliskiren /10 mg amlodipine tablets: Non-scored brown yellow, ovaloid convex shaped film-coated tablet with a beveled edge with debossing “T12” on one side and “NVR” on the reverse side of the tablet.
Do not use aliskiren with ARBs or ACEIs in patients with diabetes [see Warnings and Precautions (5.2), Clinical Studies (14.2)].
Tekamlo is contraindicated in patients with known hypersensitivity to any of the components.
5.1 Fetal Toxicity
Pregnancy Category D
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Tekamlo as soon as possible [see Use in Specific Populations (8.1)].
5.2 Renal Impairment/Hyperkalemia/Hypotension when Tekamlo is given in combination with ARBs or ACEIs
Tekamlo is contraindicated in patients with diabetes who are receiving ARBs or ACEIs because of the increased risk of renal impairment, hyperkalemia, and hypotension [see Contraindications (4) and Clinical Studies (14.2)].
Avoid use of Tekamlo with ARBs or ACEIs in patients with moderate renal impairment (GFR <60 ml/min).
5.3 Anaphylactic Reactions and Head and Neck Angioedema
Aliskiren
Hypersensitivity reactions such as anaphylactic reactions and angioedema of the face, extremities, lips, tongue, glottis and/or larynx have been reported in patients treated with aliskiren and has necessitated hospitalization and intubation. This may occur at any time during treatment and has occurred in patients with and without a history of angioedema with ACE inhibitors or angiotensin receptor antagonists. Anaphylactic reactions have been reported from post-marketing experience with unknown frequency. If angioedema involves the throat, tongue, glottis or larynx, or if the patient has a history of upper respiratory surgery, airway obstruction may occur and be fatal. Patients who experience these effects, even without respiratory distress, require prolonged observation and appropriate monitoring measures since treatment with antihistamines and corticosteroids may not be sufficient to prevent respiratory involvement. Prompt administration of subcutaneous epinephrine solution 1:1000 (0.3 to 0.5 ml) and measures to ensure a patent airway may be necessary.
Discontinue Tekamlo immediately in patients who develop anaphylactic reactions or angioedema and do not readminister.
5.4 Hypotension
Symptomatic hypotension may occur after initiation of treatment with Tekamlo in patients with marked volume depletion, patients with salt depletion, or with combined use of aliskiren and other agents acting on the renin-angiotensin-aldosterone system. The volume or salt depletion should be corrected prior to administration of Tekamlo, or the treatment should start under close medical supervision.
A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized.
Amlodipine besylate
Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. Because of the gradual onset of action, acute hypotension is unlikely.
5.5 Risk of Myocardial Infarction or Increased Angina
Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of amlodipine, particularly with severe obstructive coronary artery disease.
5.6 Impaired Renal Function
Monitor renal function periodically in patients treated with Tekamlo. Changes in renal function, including acute renal failure, can be caused by drugs that affect the renin-angiotensin-aldosterone system. Patients whose renal function may depend in part on the activity of the renin-angiotensin-aldosterone system (e.g., patients with renal artery stenosis, severe heart failure, post-myocardial infarction or volume depletion) or patients receiving ARB, ACEI or non-steroidal anti-inflammatory (NSAID) therapy may be at particular risk for developing acute renal failure on Tekamlo [see Contraindications (4), Warnings and Precautions (5.2), Clinical Studies (14.2)]. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function.
5.7 Cyclosporine or Itraconazole
Aliskiren
When aliskiren was given with cyclosporine or itraconazole, the blood concentrations of aliskiren were significantly increased. Avoid concomitant use of aliskiren with cyclosporine or intraconazole [see Drug Interactions (7)].
5.8 Hyperkalemia
Aliskiren
Monitor serum potassium periodically in patients receiving aliskiren. Drugs that affect the renin-angiotensin-aldosterone system can cause hyperkalemia. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes, combination use with ARBs or ACEI [see Contraindications (4), Warnings and Precautions (5.2), and Clinical Studies (14.2)], NSAIDs, or potassium supplements or potassium sparing diuretics.
6.1 Clinical Studies Experience
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Risk of fetal/neonatal morbidity and mortality [
see Warnings and Precautions (5.1)
]
- Head and neck angioedema [
see Warnings and Precautions (5.3)
]
- Hypotension [ see Warnings and Precautions (5.4) ]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
Tekamlo
Tekamlo has been evaluated for safety in more than 2800 patients, including 372 patients for 1 year or longer.
In a placebo-controlled study, there were 51% males, 62% Caucasians, 20% Blacks, 18% Hispanics, and 17% who were over 65 years of age. In this study, the overall incidence of adverse events on therapy with Tekamlo was similar to the individual components. Discontinuation of therapy due to a clinical adverse event in this study occurred in 1.7% of patients treated with Tekamlo (2.2% in the highest dose group) versus 1.5% of patients given placebo.
Peripheral edema is a known, dose-dependent adverse effect of amlodipine. The incidence of peripheral edema for Tekamlo in short-term double-blind placebo-controlled studies was lower than or equal to that of the corresponding amlodipine doses.
The adverse event in a placebo-controlled trial that occurred in at least 2% of patients treated with Tekamlo and at a higher incidence than placebo was peripheral edema (6.2% versus 1.0%). The incidence rate of peripheral edema at high dose was 8.9%.
In a long-term safety trial, the safety profile of adverse events was similar to that seen in the short-term controlled trials.
Aliskiren
Aliskiren has been evaluated for safety in 6460 patients, including 1740 treated for longer than 6 months, and 1250 for longer than 1 year. In placebo-controlled clinical trials, discontinuation of therapy because of a clinical adverse event, including uncontrolled hypertension, occurred in 2.2% of patients treated with aliskiren versus 3.5% of patients given placebo. These data do not include information from the ALTITUDE study which evaluated the use of aliskiren in combination with ARBs or ACEI [see Contraindications (4), Warnings and Precautions (5.2), and Clinical Studies (14.2)].
Two cases of angioedema with respiratory symptoms were reported with aliskiren use in the clinical studies. Two other cases of periorbital edema without respiratory symptoms were reported as possible angioedema and resulted in discontinuation. The rate of these angioedema cases in the completed studies was 0.06%.
In addition, 26 other cases of edema involving the face, hands, or whole body were reported with aliskiren use, including 4 leading to discontinuation.
In the placebo-controlled studies, however, the incidence of edema involving the face, hands, or whole body was 0.4% with aliskiren compared with 0.5% with placebo. In a long-term active-controlled study with aliskiren and HCTZ arms, the incidence of edema involving the face, hands, or whole body was 0.4% in both treatment arms.
Aliskiren produces dose-related gastrointestinal (GI) adverse reactions. Diarrhea was reported by 2.3% of patients at 300 mg, compared to 1.2% in placebo patients. In women and the elderly (age ≥65) increases in diarrhea rates were evident starting at a dose of 150 mg daily, with rates for these subgroups at 150 mg similar to those seen at 300 mg for men or younger patients (all rates about 2%). Other GI symptoms included abdominal pain, dyspepsia, and gastroesophageal reflux, although increased rates for abdominal pain and dyspepsia were distinguished from placebo only at 600 mg daily. Diarrhea and other GI symptoms were typically mild and rarely led to discontinuation.
Aliskiren was associated with a slight increase in cough in the placebo-controlled studies (1.1% for any aliskiren use versus 0.6% for placebo). In active-controlled trials with ACE inhibitor (ramipril, lisinopril) arms, the rates of cough for the aliskiren arms were about one-third to one-half the rates in the ACE inhibitor arms.
Other adverse reactions with increased rates for aliskiren compared to placebo included rash (1% versus 0.3%), elevated uric acid (0.4% versus 0.1%), gout (0.2% versus 0.1%), and renal stones (0.2% versus 0%).
Single episodes of tonic-clonic seizures with loss of consciousness were reported in two patients treated with aliskiren in the clinical trials. One patient had predisposing causes for seizures and had a negative electroencephalogram (EEG) and cerebral imaging following the seizures; for the other patient, EEG and imaging results were not reported. Aliskiren was discontinued and there was no rechallenge in either case.
No clinically meaningful changes in vital signs or in ECG (including QTc interval) were observed in patients treated with aliskiren.
Amlodipine besylate
Amlodipine (Norvasc®) has been evaluated for safety in more than 11,000 patients in U.S. and foreign clinical trials. Other adverse events that have been reported <1% but >0.1% of patients in controlled clinical trials or under conditions of open trials or marketing experience where a causal relationship is uncertain were:
Cardiovascular : arrhythmia (including ventricular tachycardia and atrial fibrillation), bradycardia, chest pain, peripheral ischemia, syncope, postural hypotension, vasculitis
Central and Peripheral Nervous System : neuropathy peripheral, paresthesia, tremor, vertigo
Gastrointestinal: anorexia, constipation, dyspepsia,** dysphagia, diarrhea, flatulence, pancreatitis, vomiting, gingival hyperplasia
General: allergic reaction, asthenia,** back pain, hot flushes, malaise, pain, rigors, weight gain, weight decrease
Musculoskeletal System : arthralgia, arthrosis, muscle cramps,** myalgia
Psychiatric : sexual dysfunction (male** and female), insomnia, nervousness, depression, abnormal dreams, anxiety, depersonalization
Respiratory System: dyspnea, epistaxis
Skin and Appendages : angioedema, erythema multiforme, pruritus,** rash,** rash erythematous, rash maculopapular
**These events occurred in less than 1% in placebo-controlled trials, but the incidence of these side effects was between 1% and 2% in all multiple dose studies.
Special Senses : abnormal vision, conjunctivitis, diplopia, eye pain, tinnitus
Urinary System : micturition frequency, micturition disorder, nocturia
Autonomic Nervous System : dry mouth, sweating increased
Metabolic and Nutritional : hyperglycemia, thirst
Hemopoietic : leukopenia, purpura, thrombocytopenia
Other events reported with amlodipine at a frequency of ≤0.1% of patients include: cardiac failure, pulse irregularity, extrasystoles, skin discoloration, urticaria, skin dryness, alopecia, dermatitis, muscle weakness, twitching, ataxia, hypertonia, migraine, cold and clammy skin, apathy, agitation, amnesia, gastritis, increased appetite, loose stools, rhinitis, dysuria, polyuria, parosmia, taste perversion, abnormal visual accommodation, and xerophthalmia. Other reactions occurred sporadically and cannot be distinguished from medications or concurrent disease states such as myocardial infarction and angina.
Clinical Laboratory Test Abnormalities
RBC count, hemoglobin and hematocrit : Small mean changes from baseline were seen in RBC count, hemoglobin and hematocrit in patients treated with both Tekamlo and aliskiren monotherapy. This effect is also seen with other agents acting on the renin angiotensin system. In aliskiren monotherapy trials these decreases led to slight increases in rates of anemia compared to placebo (0.1% for any aliskiren use, 0.3% for aliskiren 600 mg daily, vs. 0% for placebo). No patients discontinued due to anemia.
Blood Urea Nitrogen (BUN)/Creatinine: In patients with hypertension not concomitantly treated with an ARB or ACEI, elevations in BUN (> 40 mg/dL) and creatinine (>2.0 mg/dL) in patients treated with Tekamlo were <1.0%.
Serum Potassium: In patients with hypertension not concomitantly treated with an ARB or ACEI, increases in serum potassium >5.5 mEq/L were infrequent (0.9% compared to 0.6% with placebo) [see Contraindications (4) and Warnings and Precautions (5.10)].
6.2 Post-marketing Experience
The following adverse reactions have been identified during postapproval use of either aliskiren or amlodipine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish a causal relationship to drug exposure:
Hypersensitivity: angioedema requiring airway management and hospitalization
Aliskiren: Peripheral edema, severe cutaneous adverse reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis, urticaria, hepatic enzyme increase with clinical symptoms of hepatic dysfunction, pruritus, erythema
Hypersensitivity: anaphylactic reactions and angioedema requiring airway management and hospitalization
Amlodipine: The following postmarketing event has been reported infrequently where a causal relationship is uncertain: gynecomastia. In postmarketing experience, jaundice and hepatic enzyme elevations (mostly consistent with cholestasis or hepatitis), in some cases severe enough to require hospitalization, have been reported in association with use of amlodipine.
No drug interaction studies have been conducted with Tekamlo and other drugs, although studies with the individual aliskiren and amlodipine besylate components are described below.
Aliskiren
Cyclosporine: Avoid co-administration of cyclosporine with aliskiren.
Itraconazole: Avoid co-administration of itraconazole with aliskiren [see Clinical Pharmacology (12.3].
Non-Steroidal Anti-Inflammatory Agents(NSAIDs) including selective Cyclooxygenase-2 inhibitors (COX-2 inhibitors): In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors with agents that affect the renin-angiotensin-aldosterone system, including aliskiren, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving aliskiren and NSAID therapy.
The antihypertensive effect of aliskiren may be attenuated by NSAIDs.
Dual Blockade of the renin-angiotensin-aldosterone system: The concomitant use of aliskiren with other agents acting on the renin-angiotensin-aldosterone system such as ACEIs or ARBs is associated with an increased risk of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Monitor blood pressure, renal function, and electrolytes in patients on aliskiren and other agents that affect the renin-angiotensin-aldosterone system [see Warnings and Precautions (5.4, 5.6, 5.8)].
The concomitant use of aliskiren with an ARB or an ACEI in diabetic patients is contraindicated and should be avoided in patients with moderate renal impairment [see Contraindications (4) and Warnings and Precautions (5.2)].
Furosemide: Oral co-administration of aliskiren and furosemide reduced exposure to furosemide. Monitor diuretic effects when furosemide is co-administered with aliskiren.
Amlodipine besylate
Simvastatin: Co-administration of simvastatin with amlodipine increases the systemic exposure of simvastatin. Limit the dose of simvastatin in patients on amlodipine to 20 mg daily.
CYP3A4 Inhibitors: Co-administration with CYP3A inhibitors (moderate and strong) result in increased systemic exposure to amlodipine warranting dose reduction. Monitor for symptoms of hypotension and edema when amlodipine is co-administered with CYP3A4 inhibitors to determine the need for dose adjustment.
CYP3A4 Inducers: No information is available on the quantitative effects of CYP3A4 inducers on amlodipine. Blood pressure should be monitored when amlodipine is co-administered with CYP3A4 inducers.
8.1 Pregnancy
Pregnancy Category D
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Tekamlo as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Tekamlo, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to Tekamlo for hypotension, oliguria, and hyperkalemia. [see Use in Specific Populations (8.4)]
Animal Data
No reproductive toxicity studies have been conducted with the combination of aliskiren and amlodipine besylate. However, these studies have been conducted for aliskiren and amlodipine besylate alone.
Ali sk iren
In developmental toxicity studies, pregnant rats and rabbits received oral aliskiren hemifumarate during organogenesis at doses up to 20 and 7 times the maximum recommended human dose (MRHD) based on body surface area (mg/m2), respectively, in rats and rabbits. (Actual animal doses were up to 600 mg/kg/day in rats and up to 100 mg/kg/day in rabbits.) No teratogenicity was observed; however, fetal birth weight was decreased in rabbits at doses 3.2 times the MRHD based on body surface area (mg/m2). Aliskiren was present in placentas, amniotic fluid and fetuses of pregnant rabbits.
Amlodipine
No evidence of teratogenicity or embryo/fetal toxicity was found when pregnant rats and rabbits were treated orally with amlodipine maleate at doses up to 10 mg amlodipine/kg/day during their respective periods of major organogenesis. However, litter size was significantly decreased (by about 50%) and the number of intrauterine deaths was significantly increased (about 5-fold). Amlodipine has been shown to prolong both the gestation period and the duration of labor in rats at this dose.
8.3 Nursing Mothers
It is not known whether aliskiren or amlodipine is excreted in human milk. Both aliskiren and amlodipine are secreted in the milk of lactating rats. Because of the potential for serious adverse reactions in human milk-fed infants from Tekamlo, a decision should be made whether to discontinue nursing or discontinue Tekamlo, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness of Tekamlo in pediatric patients have not been established.
Neonates with a history of in utero exposure to Tekamlo:
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
8.5 Geriatric Use
Exposure to aliskiren and amlodipine is increased in elderly patients, thus consider lower initial doses of Tekamlo [see Clinical Pharmacology (12.3)].
In the short-term controlled clinical trials of Tekamlo, 17% of patients treated with Tekamlo were ≥ 65 years. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
Exposure to amlodipine is increased in patients with hepatic insufficiency, thus consider using lower doses of Tekamlo [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
There is no impact of renal function on the pharmacokinetics of aliskiren and amlodipine. However, safety and effectiveness of Tekamlo in patients with severe renal impairment (creatinine clearance <30 mL/min) have not been established as these patients were excluded in clinical trials [see Warnings and Precautions (5.6), Clinical Pharmacology (12.3) and Clinical Studies (14)].
Aliskiren
Limited data are available related to overdosage in humans. The most likely manifestation of overdosage would be hypotension. If symptomatic hypotension should occur, provide supportive treatment.
Aliskiren is poorly dialyzed. Therefore, hemodialysis is not adequate to treat aliskiren overexposure [see Clinical Pharmacology (12.3)].
Amlodipine besylate
Overdosage might be expected to cause excessive peripheral vasodilation with marked hypotension and possibly a reflex tachycardia. Marked and potentially prolonged systemic hypotension up to and including shock with fatal outcome have been reported. In humans, experience with intentional overdosage of amlodipine is limited. Single oral doses of amlodipine maleate equivalent to 40 mg amlodipine/kg and 100 mg amlodipine/kg in mice and rats, respectively, caused deaths. Single oral amlodipine maleate doses equivalent to 4 or more mg amlodipine/kg or higher in dogs (11 or more times the maximum recommended human dose on a mg/m2 basis) caused a marked peripheral vasodilation and hypotension.
If massive overdose should occur, initiate active cardiac and respiratory monitoring. Frequent blood pressure measurements are essential. Should hypotension occur, provide cardiovascular support including elevation of the extremities and the judicious administration of fluids. If hypotension remains unresponsive to these conservative measures, consider administration of vasopressors (such as phenylephrine) with attention to circulating volume and urine output. As amlodipine is highly protein bound, hemodialysis is not likely to be of benefit. Administration of activated charcoal to healthy volunteers immediately or up to two hours after ingestion of amlodipine has been shown to significantly decrease amlodipine absorption.
Tekamlo is a single tablet for oral administration of aliskiren hemifumarate (an orally active, nonpeptide, potent direct renin inhibitor) and amlodipine besylate (a dihydropyridine calcium channel blocker).
A liskiren hemifumarate
Aliskiren hemifumarate is chemically described as (2S,4S,5S,7S)-N-(2-carbamoyl-2-methylpropyl)-5-amino-4-hydroxy-2,7-diisopropyl-8-[4-methoxy-3-(3-methoxypropoxy)phenyl]-octanamide hemifumarate and its structural formula is:
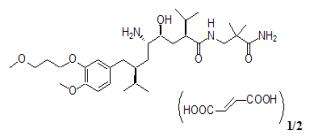
Molecular formula: C30H53N3O6 • 0.5 C4H4O4
Aliskiren hemifumarate is a white to slightly yellowish powder with a molecular weight of 609.8 (free base- 551.8). It is highly soluble in water, and freely soluble in methanol, ethanol and isopropanol.
Amlodipine besylate
Amlodipine besylate, USP is chemically described as 3-Ethyl 5-methyl (±)-2-[(2-aminoethoxy)methyl]-4-(o-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate, monobenzenesulfonate, and its structural formula is:
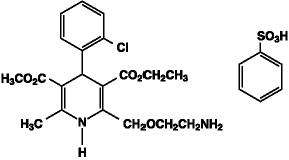
Molecular formula: C20H25CIN2O5•C6H6O3S
Amlodipine besylate is a white to pale yellow crystalline powder with a molecular weight of 567.1. It is slightly soluble in water and sparingly soluble in ethanol.
Tekamlo tablets are formulated for oral administration to contain aliskiren hemifumarate and amlodipine besylate providing for the following available combinations: 150 mg/5 mg, 150 mg/10 mg, 300 mg/5 mg and 300 mg/10 mg aliskiren/amlodipine. The inactive ingredients for all strengths of the tablets may contain colloidal silicon dioxide, crospovidone, hypromellose, iron oxide red, iron oxide yellow, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, talc, and titanium dioxide.
12.1 Mechanism of Action
Aliskiren
Renin is secreted by the kidney in response to decreases in blood volume and renal perfusion. Renin cleaves angiotensinogen to form the inactive decapeptide angiotensin I (Ang I). Ang I is converted to the active octapeptide angiotensin II (Ang II) by angiotensin-converting enzyme (ACE) and non-ACE pathways. Ang II is a powerful vasoconstrictor and leads to the release of catecholamines from the adrenal medulla and prejunctional nerve endings. It also promotes aldosterone secretion and sodium reabsorption. Together, these effects increase blood pressure. Ang II also inhibits renin release, thus providing a negative feedback to the system. This cycle, from renin through angiotensin to aldosterone and its associated negative feedback loop, is known as the renin-angiotensin-aldosterone system (RAAS). Aliskiren is a direct renin inhibitor, decreasing plasma renin activity (PRA) and inhibiting the conversion of angiotensinogen to Ang I. Whether aliskiren affects other RAAS components, e.g., ACE or non-ACE pathways, is not known.
All agents that inhibit the RAAS, including renin inhibitors, suppress the negative feedback loop, leading to a compensatory rise in plasma renin concentration. When this rise occurs during treatment with ACE inhibitors and ARBs, the result is increased levels of PRA. During treatment with aliskiren, however, the effect of increased renin levels is blocked, so that PRA, Ang I and Ang II are all reduced, whether aliskiren is used as monotherapy or in combination with other antihypertensive agents.
Amlodipine besylate
Amlodipine is a dihydropyridine calcium channel blocker that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Experimental data suggest that amlodipine binds to both dihydropyridine and nondihydropyridine binding sites. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Negative inotropic effects can be detected in vitro but such effects have not been seen in intact animals at therapeutic doses. Serum calcium concentration is not affected by amlodipine. Within the physiologic pH range, amlodipine is an ionized compound (pKa=8.6), and its kinetic interaction with the calcium channel receptor is characterized by a gradual rate of association and dissociation with the receptor binding site, resulting in a gradual onset of effect.
Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure.
Tekamlo
The effects of combined treatment of aliskiren and amlodipine arise from the actions of these two agents on different, but complementary mechanisms that regulate blood pressure, calcium channel-mediated vasoconstriction and RAAS-mediated effects on vascular tone and sodium excretion.
12.2 Pharmacodynamics
Aliskiren
PRA reductions in clinical trials ranged from approximately 50% to 80%, were not dose-related and did not correlate with blood pressure reductions. The clinical implications of the differences in effect on PRA are not known.
Amlodipine besylate
Following administration of therapeutic doses to patients with hypertension, amlodipine produces vasodilation resulting in a reduction of supine and standing blood pressures. These decreases in blood pressure are not accompanied by a significant change in heart rate or plasma catecholamine levels with chronic dosing. Although the acute intravenous administration of amlodipine decreases arterial blood pressure and increase heart rate in hemodynamic studies of patients with chronic stable angina, chronic oral administration of amlodipine in clinical trials did not lead to clinically significant changes in heart rate or blood pressures in normotensive patients with angina.
With chronic once daily administration, antihypertensive effectiveness is maintained for at least 24 hours. Plasma concentrations correlate with effect in both young and elderly patients. The magnitude of reduction in blood pressure with amlodipine is also correlated with the height of pretreatment elevation; thus, individuals with moderate hypertension (diastolic pressure 105-114 mmHg) had about 50% greater response than patients with mild hypertension (diastolic pressure 90-104 mmHg). Normotensive subjects experienced no clinically significant change in blood pressure (+1/-2 mmHg).
In hypertensive patients with normal renal function, therapeutic doses of amlodipine resulted in a decrease in renal vascular resistance and an increase in glomerular filtration rate and effective renal plasma flow without change in filtration fraction or proteinuria.
As with other calcium channel blockers, hemodynamic measurements of cardiac function at rest and during exercise (or pacing) in patients with normal ventricular function treated with amlodipine have generally demonstrated a small increase in cardiac index without significant influence on dP/dt or on left ventricular end diastolic pressure or volume. In hemodynamic studies, amlodipine has not been associated with a negative inotropic effect when administered in therapeutic dose range to intact animals and man, even when co-administered with beta-blockers to man. Similar findings, however, have been observed in normal or well-compensated patients with heart failure with agents possessing significant negative inotropic effects.
Amlodipine does not change sinoatrial nodal function or atrioventricular conduction in intact animals or man. In patients with chronic stable angina, intravenous administration of 10 mg did not significantly alter A-H and H-V conduction and sinus node recovery time after pacing. Similar results were obtained in patients receiving amlodipine and concomitant beta-blockers. In clinical studies in which amlodipine was administered in combination with beta-blockers to patients with either hypertension or angina, no adverse effects of electrocardiographic parameters were observed. In clinical trials with angina patients alone, amlodipine therapy did not alter electrocardiographic intervals or produce higher degrees of AV blocks.
Amlodipine has indications other than hypertension which can be found in the Norvasc® package insert.
Tekamlo
In a placebo-controlled study in hypertensive patients, amlodipine was associated with an increase in PRA (59-73% increase) whereas aliskiren monotherapy was associated with a 61-68% reduction in PRA. Aliskiren in combination with amlodipine reduced PRA (55-68% reduction).
12.3 Pharmacokinetics
Absorption and Distribution
Tekamlo
Following oral administration of the aliskiren/amlodipine combination tablets, the median peak plasma concentration times are within 3.0 hours for aliskiren and 8.0 hours for amlodipine. The rate and extent of absorption of aliskiren and amlodipine from Tekamlo are the same as when administered as individual tablets. When taken with food, mean AUC and Cmax of aliskiren are decreased by 79% and 90%, respectively, while there is no impact of food on the AUC and Cmax of amlodipine.
Aliskiren
Aliskiren is poorly absorbed (bioavailability about 2.5%) with an accumulation half life of about 24 hours. Steady state blood levels are reached in about 7-8 days. Following oral administration, peak plasma concentrations of aliskiren are reached within 1-3 hours. When taken with a high fat meal, mean AUC and Cmax of aliskiren are decreased by 71% and 85% respectively. In the clinical trials, aliskiren was administered without a fixed relation to meals.
Amlodipine besylate
Peak plasma concentrations of amlodipine are reached 6-12 hours after an oral administration of amlodipine. Absolute bioavailability has been estimated to be between 64% and 90%. The bioavailability of amlodipine is not altered by the presence of food.
The apparent volume of distribution of amlodipine is about 21 L/kg. Approximately 93% of circulating amlodipine is bound to plasma proteins in hypertensive patients.
Metabolism and Elimination
Aliskiren
About one-fourth of the absorbed dose appears in the urine as parent drug. How much of the absorbed dose is metabolized is unknown. Based on the in vitro studies, the major enzyme responsible for aliskiren metabolism appears to be CYP 3A4. Aliskiren does not inhibit the CYP450 isoenzymes (CYP 1A2, 2C8, 2C9, 2C19, 2D6, 2E1, and 3A) or induce CYP 3A4.
Transporters: Pgp (MDR1/Mdr1a/1b) was found to be the major efflux system involved in absorption and disposition of aliskiren in preclinical studies. The potential for drug interactions at the Pgp site will likely depend on the degree of inhibition of this transporter.
Drug interactions: The effect of co-administered drugs on the pharmacokinetics of aliskiren and vice versa, were studied in several single and multiple dose studies. Pharmacokinetic measures indicating the magnitude of these interactions are presented in Figure 5 (impact of co-administered drugs on aliskiren) and Figure 6 (impact on co-administered drugs).

Furosemide: In patients with heart failure, co-administration of aliskiren (300 mg/day) reduced plasma AUC and Cmax of oral furosemide (60 mg/day) by 17% and 27%, respectively, and reduced 24 hour urinary furosemide excretion by 29%. This change in exposure did not result in statistically significant difference in total urine volume and urinary sodium excretion over 24 hours. However, a transient decrease in urinary sodium excretion and urine volume effects up to 12 hours were observed when furosemide was co-administered with aliskiren 300 mg/day.
Amlodipine besylate
Amlodipine is extensively (about 90%) converted to inactive metabolites via hepatic metabolism with 10% of the parent compound and 60% of the metabolites excreted in the urine.
Elimination of amlodipine from the plasma is biphasic with a terminal elimination half-life of about 30-50 hours Steady state plasma levels are reached after once-daily dosing for 7-8 days.
Drug interactions:
Aliskiren exposure is increased slightly (AUC increased 29%) when aliskiren is co-administered with amlodipine, while amlodipine exposure remains unchanged when co-administered with aliskiren. The slight exposure increase of aliskiren in the presence of amlodipine is not clinically relevant.
In vitro data in human plasma indicate that amlodipine has no effect on the protein binding of digoxin, phenytoin, warfarin, and indomethacin.
Cimetidine: Co-administration of amlodipine with cimetidine did not alter the pharmacokinetics of amlodipine.
Grapefruit juice: Co-administration of 240 mL of grapefruit juice with a single oral dose of amlodipine 10 mg in 20 healthy volunteers had no significant effect on the pharmacokinetics of amlodipine.
Maalox® (antacid): Co-administration of the antacid Maalox with a single dose of amlodipine had no significant effect on the pharmacokinetics of amlodipine.
Sildenafil: A single 100 mg dose of sildenafil in subjects with essential hypertension had no effect on the pharmacokinetic parameters of amlodipine. When amlodipine and sildenafil were used in combination, each agent independently exerted its own blood pressure lowering effect.
Atorvastatin: Co-administration of multiple 10 mg doses of amlodipine with 80 mg of atorvastatin resulted in no significant change in the steady-state pharmacokinetic parameters of atorvastatin.
Digoxin: Co-administration of amlodipine with digoxin did not change serum digoxin levels or digoxin renal clearance in normal volunteers.
Ethanol (alcohol): Single and multiple 10 mg doses of amlodipine had no significant effect on the pharmacokinetics of ethanol.
Warfarin: Co-administration of amlodipine with warfarin did not change the warfarin prothrombin response time.
Simvastatin: Co-administration of multiple doses of 10 mg of amlodipine with 80 mg simvastatin resulted in a 77% increase in exposure to simvastatin compared to simvastatin alone.
CYP3A inhibitors: Co-administration of a 180 mg daily dose of diltiazem with 5 mg amlodipine in elderly hypertensive patients resulted in a 60% increase in amlodipine systemic exposure. Erythromycin co-administration in healthy volunteers did not significantly change amlodipine systemic exposure. However, strong inhibitors of CYP3A4 (e.g. ketoconazole, itraconazole, ritonavir) may increase the plasma concentrations of amlodipine to a greater extent.
Special Populations
P ediatric Patients
The pharmacokinetics of Tekamlo have not been investigated in patients <18 years of age.
Geriatric Patients
Impact of aging on aliskiren pharmacokinetics has been assessed. When compared to young adults (18-40 years), aliskiren mean AUC and Cmax in elderly subjects (> 65 years) are increased by 57% and 28%, respectively. In the elderly, clearance of amlodipine is decreased with resulting increases in peak plasma levels, elimination half-life and area-under-the-plasma-concentration curve [see Use in Specific Populations (8.5)].
Race
With Tekamlo, pharmacokinetic differences due to race have not been studied. The pharmacokinetic differences among Blacks, Caucasians, and Japanese are minimal with aliskiren therapy.
Hepatic Impairment
The pharmacokinetics of aliskiren is not significantly affected in patients with mild-to-severe liver disease. Patients with hepatic insufficiency have decreased clearance of amlodipine with resulting increase in AUC of approximately 40%-60% [see Use in Specific Populations (8.6)].
Renal Impairment
The pharmacokinetics of aliskiren was evaluated in patients with varying degrees of renal impairment. Rate and extent of exposure (AUC and Cmax) of aliskiren in subjects with renal impairment did not show a consistent correlation with the severity of renal impairment.
The pharmacokinetics of aliskiren following administration of a single oral dose of 300 mg was evaluated in patients with End Stage Renal Disease (ESRD) undergoing hemodialysis. When compared to healthy subjects, changes in the rate and extent of aliskiren exposure (Cmax and AUC) in ESRD patients undergoing hemodialysis was not clinically significant. Timing of hemodialysis did not significantly alter the pharmacokinetics of aliskiren in ESRD patients.
The pharmacokinetics of amlodipine is not significantly influenced by renal impairment [see Warnings and Precautions (5.6) and Use in Specific Populations (8.7)].
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with Aliskiren hemifumarate and Amlodipine besylate
No carcinogenicity, mutagenicity or fertility studies have been conducted with the combination of aliskiren hemifumarate and amlodipine besylate. However, these studies have been conducted for aliskiren hemifumarate and amlodipine besylate alone.
Studies with Aliskiren hemifumarate
Carcinogenic potential was assessed in a 2-year rat study and a 6-month transgenic (rasH2) mouse study with aliskiren hemifumarate at oral doses of up to 1500 mg aliskiren/kg/day. Although there were no statistically significant increases in tumor incidence associated with exposure to aliskiren, mucosal epithelial hyperplasia (with or without erosion/ulceration) was observed in the lower gastrointestinal tract at doses of 750 or more mg/kg/day in both species, with a colonic adenoma identified in one rat and a cecal adenocarcinoma identified in another, rare tumors in the strain of rat studied. On a systemic exposure (AUC0-24hr) basis, 1500 mg/kg/day in the rat is about 4 times and in the mouse about 1.5 times the maximum recommended human dose (300 mg aliskiren/day). Mucosal hyperplasia in the cecum or colon of rats was also observed at doses of 250 mg/kg/day (the lowest tested dose) as well as at higher doses in 4- and 13-week studies.
Aliskiren hemifumarate was devoid of genotoxic potential in the Ames reverse mutation assay with S. typhimurium and E. coli, the in vitro Chinese hamster ovary cell chromosomal aberration assay, the in vitro Chinese hamster V79 cell gene mutation test and the in vivo rat bone marrow micronucleus assay.
Fertility of male and female rats was unaffected at doses of up to aliskiren 250 mg/kg/day (8 times the maximum recommended human dose of aliskiren 300 mg/60 kg on a mg/m2 basis).
Studies with Amlodipine besylate
Rats and mice treated with amlodipine maleate in the diet for up to two years, at concentrations calculated to provide daily dosage levels of 0.5, 1.25, and 2.5 mg amlodipine/kg/day, showed no evidence of a carcinogenic effect of the drug. For the mouse, the highest dose was, on mg/m2 basis, similar to the maximum recommended human dose (MRHD) of 10 mg amlodipine/day. For the rat, the highest dose was, on a mg/m2 basis, about twice the MRHD.
Mutagenicity studies conducted with amlodipine maleate revealed no drug-related effects at either the gene or chromosome level.
There was no effect on the fertility of rats treated orally with amlodipine maleate (males for 64 days and females for 14 days prior to mating) at doses of up to 10 mg amlodipine/kg/day (about 8 times the MRHD of 10 mg/day on a mg/m2 basis).
13.2 Animal Toxicology and/or Pharmacology
Preclinical safety studies have demonstrated that the combination of aliskiren hemifumarate and amlodipine besylate was well tolerated in rats. The findings from the 2- and 13-week oral toxicity studies in rats were consistent with those of aliskiren hemifumarate and amlodipine besylate when both drugs were administered alone. There were no new toxicities or increased severity of the toxicities which were associated with either component.
Animal reproductive and developmental toxicology findings are described elsewhere [see Use in Specific Populations (8.1)].
14.1 Tekamlo
Tekamlo was studied in a total of 5549 patients with mild to moderate hypertension (diastolic blood pressure between 90 mmHg and 109 mmHg).
Aliskiren 150 mg and 300 mg and amlodipine besylate 5 mg and 10 mg were studied alone and in combination in an 8-week, randomized, double-blind, placebo-controlled, multifactorial study comparing the combinations 150 mg/5 mg, 150 mg/10 mg, 300 mg/5 mg and 300 mg/10 mg of aliskiren and amlodipine with their components and placebo. The combination of aliskiren and amlodipine resulted in placebo-adjusted decreases in systolic/diastolic blood pressure at trough of 14-17/9-11 mmHg compared to 4-9/3-5 mmHg for aliskiren alone and 9-14/6-8 mmHg for amlodipine alone.
Treatment with Tekamlo resulted overall in significantly greater reductions in diastolic and systolic blood pressure compared to the respective monotherapy components.
The antihypertensive effect of Tekamlo was similar in patients with and without diabetes, obese and non-obese patients, in patients ≥65 years of age and <65 years of age, and in women and men.
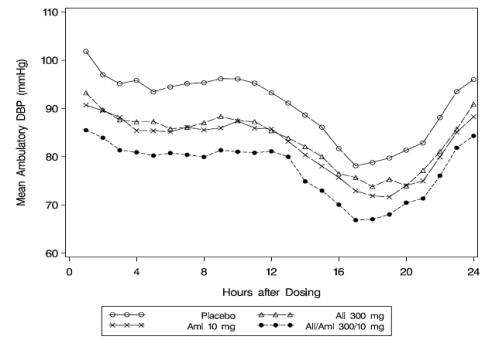
A subgroup of 819 patients was studied with ambulatory blood pressure monitoring. The blood pressure lowering effect in the aliskiren/amlodipine group was maintained throughout the 24-hour period (see Figure 7 and Figure 8).
Two additional double-blind, active-controlled studies of similar design were conducted in which Tekamlo was administered as initial therapy in patients with moderate to severe hypertension (SBP 160-200 mmHg). Patients were randomized to receive either combination aliskiren/amlodipine or amlodipine monotherapy. The initial dose of aliskiren/amlodipine was 150 mg/5 mg for 1 week with forced titration to 300 mg/10 mg for 7 weeks. The initial dose of amlodipine was 5 mg for 1 week with forced titration to 10 mg for 7 weeks. In one study of 443 Black patients, at the primary endpoint of 8 weeks, the treatment difference between aliskiren/amlodipine and amlodipine was 5.2/3.8 mmHg. In the other study of 484 patients, at the primary endpoint of 8 weeks, the treatment difference between aliskiren/amlodipine and amlodipine was 7.1/3.8 mmHg.
The blood pressure lowering effects of Tekamlo are largely attained within 1-2 weeks.
There are no trials of the Tekamlo combination tablet demonstrating reductions in cardiovascular risk in patients with hypertension, but the amlodipine component has demonstrated such benefits.
14.2 Aliskiren in Patients with Diabetes treated with ARB or ACEI (ALTITUDE study)
Patients with diabetes with renal disease (defined either by the presence of albuminuria or reduced GFR) were randomized to aliskiren 300 mg daily (n=4296) or placebo (n=4310). All patients were receiving background therapy with an ARB or ACEI. The primary efficacy outcome was the time to the first event of the primary composite endpoint consisting of cardiovascular death, resuscitated sudden death, non-fatal myocardial infarction, non-fatal stroke, unplanned hospitalization for heart failure, onset of end stage renal disease, renal death, and doubling of serum creatinine concentration from baseline sustained for at least one month. After a median follow up of about 32 months, the trial was terminated early for lack of efficacy. Higher risk of renal impairment, hypotension and hyperkalemia was observed in aliskiren compared to placebo treated patients, as shown in the table below.
| †renal failure, renal failure acute, renal failure chronic, renal impairment | ||||
| ††dizziness, dizziness postural, hypotension, orthostatic hypotension, presyncope, syncope | ||||
| ††† Given the variable baseline potassium levels of patients with renal insufficiency on dual RAAS therapy, the reporting of adverse event of hyperkalemia was at the discretion of the investigator. | ||||
| * A Serious Adverse Event (SAE) is defined as: an event which is fatal or life-threatening, results in persistent or significant disability/incapacity, constitutes a congenital anomaly/birth defect, requires inpatient hospitalization or prolongation of existing hospitalization, or is medically significant (i.e. defined as an event that jeopardizes the patient or may require medical or surgical intervention to prevent one of the outcomes previously listed). | ||||
| Aliskiren N=4272 |
Placebo N=4285 |
|||
| Serious Adverse Events* (%) | Adverse Events (%) | Serious Adverse Events* (%) | Adverse Events (%) | |
| Renal impairment † | 5.7 | 14.5 | 4.3 | 12.4 |
| Hypotension †† | 2.3 | 19.9 | 1.9 | 16.3 |
| Hyperkalemia ††† | 1.0 | 38.9 | 0.5 | 28.8 |
The risk of stroke (3.4% aliskiren vs 2.7% placebo) and death (8.4% aliskiren vs. 8.0% placebo) were also numerically higher in aliskiren treated patients.
16 HOW SUPPLIED/STORAGE AND HANDLING
Tekamlo (aliskiren and amlodipine) is supplied as follows:
150 mg aliskiren/5 mg amlodipine Tablets - Non-scored light yellow, ovaloid convex-shaped, film-coated tablet with a beveled edge with debossing “T2” on one side and “NVR” on the reverse side of the tablet. The tablet dimensions are approximately 16 x 6.3 mm.
150 mg aliskiren/10 mg amlodipine Tablets - Non-scored yellow, ovaloid convex shaped, film-coated tablet with a beveled edge with debossing “T7” on one side and “NVR” on the reverse side of the tablet. The tablet dimensions are approximately 16 x 6.3 mm.
300 mg aliskiren/5 mg amlodipine Tablets - Non-scored dark yellow, ovaloid convex-shaped, film-coated tablet with a beveled edge with debossing “T11” on one side and “NVR” on the reverse side of the tablet. The tablet dimensions are approximately 21 x 8.3 mm.
300 mg aliskiren/10 mg amlodipine Tablets - Non-scored brown yellow, ovaloid convex shaped, film-coated tablet with a beveled edge with debossing “T12” on one side and “NVR” on the reverse side of the tablet. The tablet dimensions are approximately 21 x 8.3 mm.
All strengths are packaged in bottles and unit-dose blister packages (10 strips of 10 tablets) as described below.
| Tablet | Color | Debossed | Debossed | NDC 0078- XXXX-XX | ||
| Aliskiren hemifumarate /amlodipine besylate | Side 1 | Side 2 | Bottle of 30 | Bottle of 90 | Blister Packages of 100 | |
| 150 mg/5 mg | Light yellow | T2 | NVR | 0603-15 | 0603-34 | 0603-35 |
| 150 mg/10 mg | Yellow | T7 | NVR | 0604-15 | 0604-34 | 0604-35 |
| 300 mg/5 mg | Dark yellow | T11 | NVR | 0605-15 | 0605-34 | 0605-35 |
| 300 mg/10 mg | Brown yellow | T12 | NVR | 0606-15 | 0606-34 | 0606-35 |
Storage
Store at 25ºC (77ºF); excursions permitted to 15-30ºC (59-86ºF) in original container.
Protect from heat and moisture.
Dispense in original container.
See FDA-Approved Patient Labeling (Patient Information)
Healthcare professionals should instruct their patients to read the Patient Package Insert before starting Tekamlo and to reread each time the prescription is renewed. Patients should be instructed to inform their doctor or pharmacist if they develop any unusual symptom, or if any known symptom persists or worsens.
Pregnancy
Female patients of childbearing age should be told about the consequences of exposure to Tekamlo during pregnancy. Discuss treatment options with women planning to become pregnant. Patients should be asked to report pregnancies to their physician as soon as possible.
Symptomatic Hypotension
Caution patients receiving Tekamlo that lightheadedness can occur, especially during the first days of therapy, and that it should be reported to the prescribing physician. Tell patients that if syncope occurs, discontinue Tekamlo until the physician has been consulted.
Caution all patients that inadequate fluid intake, excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure, with the same consequences of lightheadedness and possible syncope.
Anaphylactic Reactions and Angioedema
Patients should be advised and told to report immediately any signs or symptoms suggesting a severe allergic reaction (difficulty breathing or swallowing, tightness of the chest, hives, general rash, swelling, itching, dizziness, vomiting, or abdominal pain) or angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) and to take no more drug until they have consulted with the prescribing physician. Angioedema, including laryngeal edema, may occur at any time during treatment with Tekamlo.
Potassium Supplements
Tell patients receiving Tekamlo not to use potassium supplements or salt substitutes containing potassium without consulting the prescribing physician.
Relationship to Meals
Patients should establish a routine pattern for taking Tekamlo with regard to meals. High-fat meals decrease absorption substantially.
T2014-22
March 2014
FDA-Approved Patient Labeling
Patient Information
Tekamlo
TM
(
těk’-ăm-lō
)
Tekamlo
(
aliskiren
and
amlodipine
)
Tablets
Read the Patient Information that comes with Tekamlo before you start taking it and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your condition and treatment. If you have any questions about Tekamlo, ask your doctor or pharmacist.
What is the most important information I should know about Tekamlo?
Tekamlo can cause harm or death to an unborn baby. Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant. If you get pregnant while taking Tekamlo, tell your doctor right away.
What is Tekamlo?
Tekamlo is a prescription medicine that may be used:
- as the first medicine to lower your high blood pressure if your doctor decides that you are likely to need more than one medicine.
- to treat your high blood pressure when one medicine to lower your high blood pressure has not worked well enough.
- if you are already taking the medicines aliskiren and amlodipine to treat your high blood pressure.
Tekamlo contains:
- aliskiren, a direct renin inhibitor (DRI)
- amlodipine, a calcium channel blocker (CCB)
Your doctor may prescribe other medicines for you to take along with Tekamlo to treat your high blood pressure.
It is not known if Tekamlo is safe and works in children under 18 years of age.
Who should not take Tekamlo?
- If you get pregnant, stop taking Tekamlo and call your doctor right away. If you plan to become pregnant, talk to your doctor about other treatment options for your high blood pressure.
- If you have diabetes and are taking a kind of medicine called an angiotensin-receptor-blocker or angiotensin-converting-enzyme-inhibitor.
- If you are allergic (hypersensitive) to aliskiren, amlodipine or other dihydropyridines (calcium-channel blockers, a group of medicines to lower blood pressure to which amlodipine belongs) or any of the other ingredients of Tekamlo listed at the end of this leaflet.
What should I tell my doctor before taking Tekamlo?
Before taking Tekamlo, tell your doctor if you :
- have kidney problems
- have liver problems
- have ever had an allergic reaction to another blood pressure medicine. Symptoms may include: swelling of the face, lips, tongue, throat, arms and legs, and trouble breathing.
- suffer from heart disorders or if you experienced a heart attack
- have any other medical problems
- are pregnant or planning to become pregnant. See “What is the most important information I should know about Tekamlo?”
- are breastfeeding. It is not known if Tekamlo passes into your breast milk and if it can harm your baby. You and your doctor should decide if you will take Tekamlo or breastfeed. You should not do both.
Tell your doctor about all the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. Tekamlo and certain other medicines may affect each other and cause side effects.
Especially tell your doctor if you take:
- a kind of medicine called angiotensin receptor blocker or angiotensin converting enzyme inhibitor
- water pills (also called “diuretics”)
- medicines for treating fungus or fungal infections
- cyclosporine (Gengraf®, Neoral, Sandimmune), a medicine used to suppress the immune system
- potassium-containing medicines, potassium supplements, or salt substitutes containing potassium
- simvastatin (Zocor®) or atorvastatin (Lipitor®)
- non-steroidal anti-inflammatory drugs (like ibuprofen or naproxen)
- medicines used to treat AIDS or HIV infections (such as ritonavir, indinavir)
Know your medicines. Keep a list of all your medicines. Show this list to your doctor or pharmacist when you get a new medicine. Your doctor or pharmacist will know what medicines are safe to take together.
How should I take Tekamlo?
- Take Tekamlo exactly as prescribed by your doctor. It is important to take Tekamlo every day to control your blood pressure.
- Take Tekamlo one time a day, about the same time each day.
- Take Tekamlo the same way every day, either with or without a meal.
- Your doctor may change your dose of Tekamlo if needed. Do not change the amount of Tekamlo you take without talking to your doctor.
- If you miss a dose of Tekamlo, take it as soon as you remember. If it is close to your next dose, do not take the missed dose. Just take the next dose at your regular time.
- If you take too much Tekamlo, call your doctor or a Poison Control Center, or go to the nearest hospital emergency room.
What are the possible side effects of Tekamlo?
Tekamlo may cause serious side effects:
-
Harm to an unborn baby
,
causing injury or death. See “What is the most important information I should know about Tekamlo?”
- Severe Allergic Reactions and Angioedema. Aliskiren, one of the medicines in Tekamlo, can cause difficulty breathing or swallowing, tightness of the chest, hives, general rash, swelling, itching, dizziness, vomiting, or abdominal pain (signs of a severe allergic reaction). Aliskiren can also cause swelling of your face, lips, tongue, throat, arms and legs, or the whole body (signs of angioedema). Get medical help right away and tell your doctor if you get any one or more of these symptoms. Angioedema can happen at any time while you are taking Tekamlo.
-
Low blood pressure (hypotension). Your blood pressure may get too low if you also take water pills, are on a low-salt diet, get dialysis treatments, have heart problems, or get sick with vomiting or diarrhea. Lie down if you feel faint or get dizzy. Call your doctor right away.
- Possible increased chest pain or risk of heart attack. It is rare, but when you first start taking Tekamlo or increase your dose, you may have a heart attack or your angina may get worse. If that happens, call your doctor right away or go directly to a hospital emergency room.
- Renal impairment or failure. Aliskiren, one of the medicines in Tekamlo, may cause renal disorder with symptoms such as severely decreased urine output or decreased urine output (signs of renal impairment or failure).
The most common side effects of Tekamlo include:
- Swelling of your lower legs
Common side effects of Tekamlo include:
- diarrhea
- cough
- dizziness
- flu-like symptoms
- tiredness
- high levels of potassium in the blood (hyperkalemia)
Less common side effects include rash, severe skin reactions (signs may include severe blistering of the lips, eyes or mouth, rash with fever and skin peeling) and liver disorder (signs may include nausea, loss of appetite, dark colored urine or yellowing of skin and eyes).
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of Tekamlo. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store Tekamlo?
- Store Tekamlo tablets at room temperature between 59oF to 86oF (15oC to 30oC).
- Keep the original prescription bottle and store in a dry place.
- Protect Tekamlo from heat and moisture.
Keep Tekamlo and all medicines out of the reach of children.
General information about Tekamlo
Medicines are sometimes prescribed for conditions not listed in the patient information leaflet. Do not take Tekamlo for a condition for which it was not prescribed. Do not give Tekamlo to other people, even if they have the same condition or symptoms you have. It may harm them.
This leaflet summarizes the most important information about Tekamlo. If you have questions about Tekamlo talk with your doctor. You can ask your doctor or pharmacist for information that is written for healthcare professionals.
For more information about Tekamlo, visit www.Tekamlo.com, or call 1-888-NOW-NOVA (1-888-669-6682) .
What are the ingredients in Tekamlo?
Active Ingredients: Aliskiren hemifumarate and amlodipine
Inactive ingredients: Colloidal silicon dioxide, crospovidone, hypromellose, iron oxide red, iron oxide yellow, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, talc, and titanium dioxide.
What is high blood pressure (hypertension)?
Blood pressure is the force of blood in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too much.
High blood pressure makes the heart work harder to pump blood through the body and causes damage to blood vessels. Tekamlo can help your blood vessels relax so your blood pressure is lower. Medicines that lower your blood pressure may lower your chance of having a stroke or heart attack.
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
© Novartis
T2013-109
November 2013
PRINCIPAL DISPLAY PANEL
Package Label – 150 mg /5 mg
Rx Only NDC 0078-0603-15
Tekamlo™
(aliskiren and amlodipine)
Tablets
150 mg*/5 mg* per tablet
*each tablet contains 165.8 mg of
Aliskiren hemifumarate and 6.9 mg
of amlodipine besylate
30 tablets

PRINCIPAL DISPLAY PANEL
Package Label – 150 mg /10 mg
Rx Only NDC 0078-0604-15
Tekamlo™
(aliskiren and amlodipine)
Tablets
150 mg*/10 mg* per tablet
*each tablet contains 165.8 mg of
aliskiren hemifumarate and 13.9 mg
of amlodipine besylate
30 tablets

PRINCIPAL DISPLAY PANEL
Package Label – 300 mg /5 mg
Rx Only NDC 0078-0605-15
Tekamlo™
(aliskiren and amlodipine)
Tablets
300 mg*/5 mg* per tablet
*each tablet contains 331.5 mg of
aliskiren hemifumarate and 6.9 mg
of amlodipine besylate
30 tablets

PRINCIPAL DISPLAY PANEL
Package Label – 300 mg /10 mg
Rx Only NDC 0078-0606-15
Tekamlo™
(aliskiren and amlodipine)
Tablets
300 mg*/10 mg* per tablet
*each tablet contains 331.5 mg of
aliskiren hemifumarate and 13.9 mg
of amlodipine besylate
30 tablets

TekamloAliskiren Hemifumarate and Amlodipine Besylate TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TekamloAliskiren Hemifumarate and Amlodipine Besylate TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TekamloAliskiren Hemifumarate and Amlodipine Besylate TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TekamloAliskiren Hemifumarate and Amlodipine Besylate TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||