Telazol
Telazol CIII (TILETAMINE HCl and ZOLAZEPAM HCl)
FULL PRESCRIBING INFORMATION: CONTENTS*
- CAUTION
- TELAZOL DESCRIPTION
- ACTIONS
- INDICATIONS
- TELAZOL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TELAZOL ADVERSE REACTIONS
- ADMINISTRATION AND DOSAGE
- PREPARATION OF SOLUTION
- HOW SUPPLIED
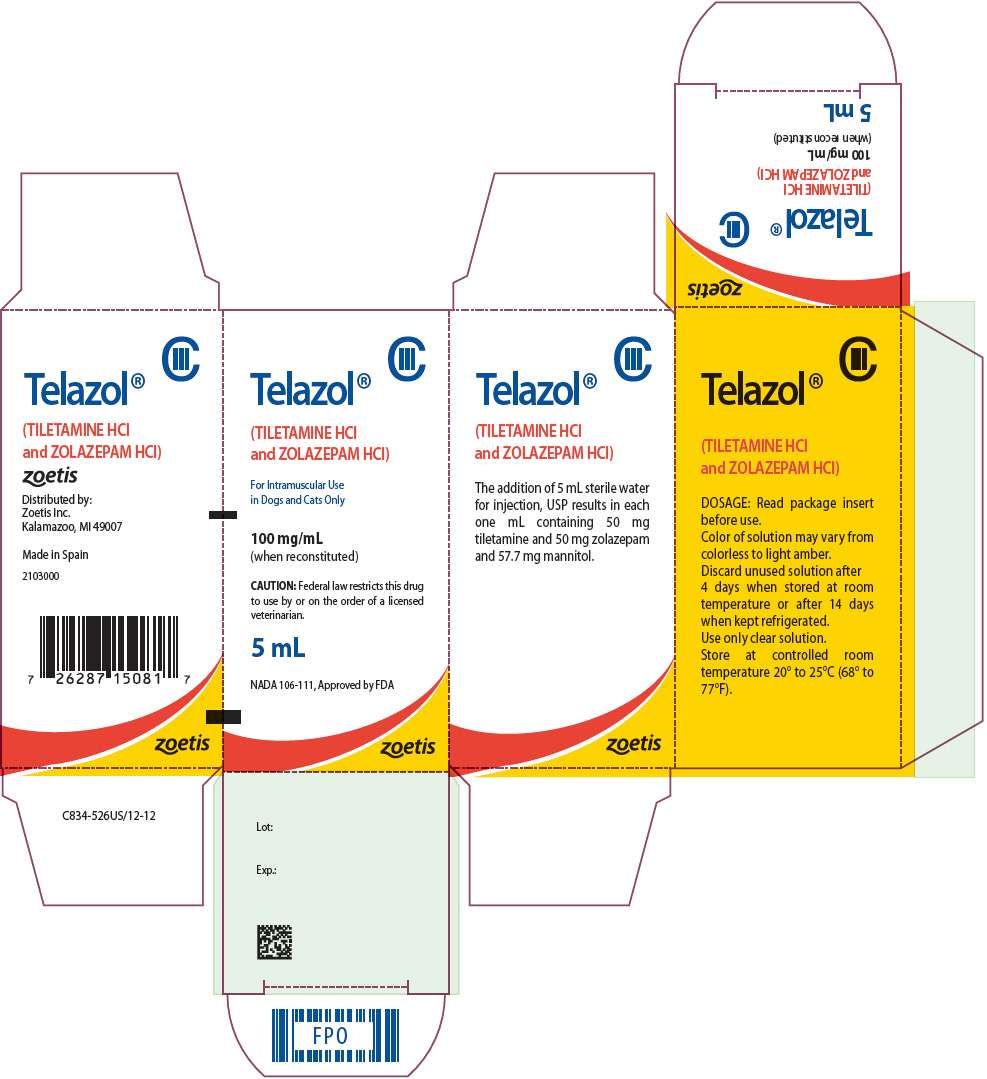
- PRINCIPAL DISPLAY PANEL - 5 mL Bottle Carton
FULL PRESCRIBING INFORMATION
NADA 106-111, Approved by FDA
For Intramuscular Use in Dogs and Cats Only
CAUTION
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
TELAZOL DESCRIPTION
TELAZOL (tiletamine HCl and zolazepam HCl) is a nonnarcotic, nonbarbiturate, injectable anesthetic agent for dogs and cats. Chemically, TELAZOL is a combination of equal parts by weight of base of tiletamine hydrochloride (2-[ethylamino]-2-[2-thienyl]-cyclohexanone hydrochloride), an arylaminocycloalkanone dissociative anesthetic, and zolazepam hydrochloride (4-[o-fluorophenyl]-6,8-dihydro-1,3,8- trimethylpyrazolo [3, 4-e] [1,4] diazepin-7 [1H]- 1-hydrochloride), a non- phenothiazine diazepinone having minor tranquilizing properties. The product is supplied sterile in vials. The addition of 5 mL diluent produces a solution containing the equivalent of 50 mg tiletamine base, 50 mg zolazepam base and 57.7 mg mannitol per milliliter. This solution has a pH of 2 to 3.5 and is recommended for deep intramuscular injection.
ACTIONS
TELAZOL is a rapid-acting anesthetic combination of tiletamine hydrochloride and zolazepam hydrochloride. Tiletamine hydrochloride is a dissociative anesthetic agent whose pharmacologic action is characterized by profound analgesia, normal pharyngeallaryngeal reflexes and cataleptoid anesthesia. The anesthetic state produced does not fit into the conventional classification of stages of anesthesia, but instead TELAZOL produces a state of unconsciousness which has been termed "dissociative" anesthesia in that it appears to selectively interrupt association pathways to the brain before producing somesthetic sensory blockade.
Cranial nerve and spinal reflexes remain active; however, these reflexes must not be confused with inadequate anesthesia. Analgesia results from apparent selective interruption of sensory inputs to the brain and usually persists after the anesthetic effect has subsided.
Protective reflexes, such as coughing and swallowing, are maintained under tiletamine anesthesia. Other reflexes, e.g., corneal, pedal, are maintained during tiletamine anesthesia, and should not be used as criteria for judging depth of anesthesia. The eyes normally remain open with the pupil dilated. It is suggested that a bland ophthalmic ointment be applied to the cornea if anesthesia is to be prolonged.
Used alone, tiletamine hydrochloride does not provide adequate muscle relaxation for abdominal surgical procedures. When combined with zolazepam hydrochloride, good muscle relaxation is generally attained during the phase of deep surgical anesthesia.
Following a single, deep intramuscular injection of TELAZOL in cats and dogs, onset of anesthetic effect usually occurs within 5 to 12 minutes. Muscle relaxation is optimum for approximately the first 20 to 25 minutes after TELAZOL is administered, and then diminishes. Recovery varies with the age and physical condition of the animal and the dose of TELAZOL administered, but usually requires several hours. Recovery is extended with multiple injections, particularly in cats.
Repeated doses increase the duration of the effect of TELAZOL but may not further diminish muscle tone. The quality of anesthesia with repeated doses varies because the ratio of the two components within the animal's body changes with each injection. This is due to the difference in the rates of metabolism and elimination of the two components. The quality of anesthesia will be improved and more predictable if the entire dose is given as a single injection rather than in several doses. The best method of evaluating the depth of TELAZOL anesthesia is to monitor the patient for deliberate conscious response to nociceptive stimuli.
Copious salivation may occur during TELAZOL anesthesia. Ptyalism may be controlled in dogs and cats by giving atropine sulfate, USP, 0.02 mg/lb (0.04 mg/kg) body weight, as concurrent medication. Exaggerated swallowing, reflex action and accumulation of saliva may give rise to vomiting and retching.
TELAZOL has a wider margin of safety in cats than in dogs. Dogs have survived repeated dosage regimens of 13.6 mg/lb (30 mg/kg) (maximum safe dose) for eight successive days.
This is approximately two times the maximum recommended therapeutic dose. Cats have survived dosage regimens of up to 32.7 mg/lb (72 mg/kg) (maximum safe dose) on alternate days for seven episodes. This is 4.6 times the maximum recommended therapeutic dose for cats. However, these reports should not obviate prudent anesthetic practices. Some degree of tolerance has been reported. This tolerance appears to be species-variable.
Cats: In cats, the duration of effect of zolazepam exceeds that of tiletamine so that as the animal recovers there is a greater degree of tranquilization than anesthetization. There is a slight lowering of blood pressure during the first hour after injection. Heart rate and electrocardiogram readings are unaffected by TELAZOL (tiletamine HCl and zolazepam HCl). Arterial pO2 levels are decreased three minutes after injection but usually return to normal within 15 to 35 minutes.
Dogs: In dogs, the duration of effect of tiletamine exceeds that of zolazepam so there is a lesser degree of tranquilization than anesthetization in this species. The total effect of TELAZOL in dogs is of shorter duration than in cats.
Following administration of TELAZOL in dogs, a marked, persistent tachycardia occurs within two minutes following either 4.5 or 9 mg/lb (10 or 20 mg/kg) TELAZOL intramuscularly. Stroke volume decreases proportionately to the increased rate at the 4.5 mg/lb (10 mg/kg) dose, with little change in net cardiac output. There is an initial increase in systolic blood pressure, with a slight drop in pressure within five minutes. The systolic blood pressure remains at this decreased level throughout the duration of the anesthetic effect. Diastolic pressure increases throughout this same period. Following a 9 mg/lb (20 mg/kg) dose of TELAZOL in dogs, the relationship between stroke volume and heart rate is disproportionate, with a resultant substantial decrease in cardiac output. Contractility and mean blood pressure are decreased, indicating direct myocardial depression. Ventricular function is adequate. During surgical manipulations, tachycardia and hypertension may be observed, and may be brought on by sympathetic reaction to painful stimuli. Epinephrine is markedly less arrhythmogenic in animals under TELAZOL anesthesia than in those under halothane anesthesia.
During TELAZOL anesthesia, the assurance of a patent airway is greatly enhanced by virtue of maintaining pharyngeal-laryngeal reflexes. During the first 15 minutes after intramuscular administration of 9 mg/lb (20 mg/kg) of TELAZOL, the respiratory rate is doubled while the tidal volume is decreased to less than one-half of control values. Arterial pO2 levels also decrease. This may be evidenced by hypoxemia and cyanosis. The pulmonary function usually returns to normal within 35 minutes after the administration of TELAZOL.
INDICATIONS
TELAZOL is indicated in cats for restraint or for anesthesia combined with muscle relaxation and in dogs for restraint and minor procedures of short duration (30 min. avg.) requiring mild to moderate analgesia. Minor surgery is considered to be laceration repair, draining of abscesses, castrations and other procedures requiring mild to moderate analgesia. (See Dogs under Administration and Dosage.)
TELAZOL CONTRAINDICATIONS
The use of TELAZOL is contraindicated in dogs and cats with pancreatic disease. TELAZOL is excreted predominantly by the kidneys. Preexistent renal pathology or impairment of renal function may be expected to result in prolonged duration of anesthesia. TELAZOL should not be used in dogs and cats with severe cardiac or pulmonary dysfunction. Because the teratogenic potential of TELAZOL is unknown, it should not be used in pregnant bitches or queens at any stage of pregnancy. Also, a study has shown that TELAZOL crosses the placental barrier and produces respiratory depression in the newborn; therefore, its use for Cesarean section is contraindicated.
WARNINGS
FOR USE IN DOGS AND CATS ONLY. The principal route of excretion of both components in the cat is the urine; therefore, TELAZOL is not recommended for use in cats suffering from renal insufficiency.
Balance studies in dogs indicated extensive biotransformation of both components with less than 4% of the dose excreted unchanged in the urine.
The safety of the use of TELAZOL (tiletamine HCl and zolazepam HCl) in pregnant animals or on reproduction has not been established. TELAZOL crosses the placental barrier and causes respiratory depression in the neonate. Phenothiazine-derivative drugs should not be used with TELAZOL because the combination produces respiratory and myocardial depression, hypotension and hypothermia. Pulmonary edema has been reported to occur in cats with the use of TELAZOL. Signs and symptoms include dyspnea, lethargy, anorexia and abnormal behavior. Deaths have been reported occasionally in severely affected individuals. Cats should be observed closely for any signs and symptoms which may suggest pulmonary edema so that appropriate therapy may be instituted.
PRECAUTIONS
The dosage of TELAZOL should be reduced in geriatric dogs and cats, in animals in debilitated condition and in animals with impairment of renal function. Death has occurred in both cats and dogs following TELAZOL administration. Preexisting pulmonary disease, renal disease (see Contraindications and Warnings) and shock were causally implicated at necropsy; however, death was drug attributable in at least one dog (of 1072) and one cat (of 1095). Cats and smaller dogs with small body masses in relation to large body surfaces should be protected from heat loss during TELAZOL anesthesia. Body temperature should be monitored, and supplemental heat may be required to control hypothermia. As with other anesthetics, it is prudent to provide for hemostasis during any surgical procedure.
During TELAZOL anesthesia, athetoid movement may occur. This athetosis should not be mistaken for lack of anesthesia nor is it indicative of lack of analgesia. Do not give additional anesthesia in an attempt to abolish the athetoid movement. Efforts to eliminate athetoid movement with additional doses of TELAZOL can result in anesthetic overdosage. TELAZOL does not abolish laryngeal, pharyngeal, pinnal, palpebral and pedal reflexes, and may not be adequate as the sole anesthetic for surgical procedures in these areas.
Endotracheal tubes are not well tolerated in connection with TELAZOL anesthesia in the cat and their use may result in impaired respiration.
After removal of the tube, normal respiration should resume.
The stimulation of surgical procedures aids in maintaining adequate ventilation. The anesthetized patient must be monitored throughout the procedure, and if cardiopulmonary problems do occur, measures must be taken to assure that alveolar ventilation and cardiovascular functions are maintained.
The eyes normally remain open with the pupils dilated. The use of a bland ophthalmic ointment is advisable to protect the corneas from desiccation. A study has indicated that the concurrent use of chloramphenicol will prolong the duration of anesthesia in cats.
Atropine (0.02 mg/lb) (0.04 mg/kg) should be used to control ptyalism.
TELAZOL ADVERSE REACTIONS
Respiratory depression may occur following administration of high doses of TELAZOL. If at any time respiration becomes excessively depressed and the animal becomes cyanotic, resuscitative measures should be instituted promptly. Adequate pulmonary ventilation using either oxygen or room air is recommended as a resuscitative measure.
Adverse reactions reported have included emesis during emergence, excessive salivation, transient apnea, vocalization, erratic recovery and prolonged recovery, excessive tracheal and bronchial secretions when atropine sulfate, USP, was not given before anesthesia, involuntary muscular twitching, hypertonicity, cyanosis, cardiac arrest, pulmonary edema and muscle rigidity during surgical procedures. Central nervous system stimulation and convulsions have also been reported. Tachycardia frequently occurs, particularly in the dog. This rise in heart rate usually lasts about 30 minutes. Either hypertension or hypotension may also occur. Insufficient anesthesia has been reported in dogs.
Death has been reported in dogs and cats following TELAZOL administration.
ADMINISTRATION AND DOSAGE
TELAZOL is well tolerated by dogs and cats and should be administered by deep intramuscular injection in prescribed doses. At high doses, recovery is usually prolonged.
There may be pain on injection. This is especially prevalent in cats.
Fasting prior to induction of general anesthesia with TELAZOL (tiletamine HCl and zolazepam HCl) is not essential; however, when preparing for elective surgery, it is advisable to withhold food for at least 12 hours prior to TELAZOL administration.
As with other injectable anesthetic agents, the individual response to TELAZOL is somewhat varied, depending upon the dose, general physical condition and age of the patient and duration of the surgical procedure. Therefore, recommendations for dosage regimens cannot be fixed absolutely. Specific dosage requirements must be determined by evaluation of the health status and condition of the patient and of the procedure to be performed.
If adequate anesthesia is not produced by the recommended dosage regimen, supplemental anesthesia or another agent is indicated. This includes the use of barbiturates and volatile anesthetics. When used concurrently with TELAZOL the dosage of these agents should be reduced.
Atropine sulfate, USP, 0.02 mg/lb (0.04 mg/ kg), should be used as concurrent medication to control ptyalism.
Dogs: In healthy dogs, an initial intramuscular dosage of 3 to 4.5 mg/lb (6.6 to 9.9 mg/kg) TELAZOL is recommended for diagnostic purposes; 4.5 to 6 mg/lb (9.9 to 13.2 mg/kg) for minor procedures of short duration, such as treatment of lacerations and wounds, castrations and other procedures requiring mild to moderate analgesia. When supplemental doses of TELAZOL are required, such individual supplemental doses should be less than the initial dose, and the total dose given (initial dose plus supplemental dose or doses) should not exceed 12 mg/lb (26.4 mg/kg). The maximum safe dose is 13.6 mg/lb (29.92 mg/kg). (See Actions.) Results from TELAZOL anesthesia in dogs will be more satisfactory if the procedures are completed within one hour and if the procedures can be completed following single dose administration. In order to maintain at least a 2× margin of safety in dogs, the use of this product is limited to procedures that call for low doses (see Indications). Studies show that there is variation in response to different dosages of TELAZOL and that low doses do not give adequate levels of anesthesia, and in some instances do not give adequate analgesia, for extensive procedures.
Cats: In healthy cats, an initial TELAZOL dosage of 4.4 to 5.4 mg/lb (9.7 to 11.9 mg/kg) is recommended for such procedures as dentistry, treatment of abscesses, foreign body removal and related types of surgery; 4.8 to 5.7 mg/lb (10.6 to 12.5 mg/kg) for minor procedures requiring mild to moderate analgesia, such as repair of lacerations, castrations and other procedures of short duration. Initial dosages of 6.5 to 7.2 mg/lb (14.3 to 15.8 mg/kg) are recommended for ovario- hysterectomy and onychectomy. When supplemental doses of TELAZOL are required, such individual supplemental doses should be given in increments that are less than the initial dose, and the total dose given (initial dose plus supplemental doses) should not exceed the maximum allowable safe dose of 32.7 mg/lb (72 mg/kg). (See Actions.)
PREPARATION OF SOLUTION
To each vial add 5 mL sterile water for injection, USP. Slight agitation will facilitate complete reconstitution. The resultant solution will contain 100 mg active ingredient per one milliliter.
Discard unused solution after 4 days when stored at room temperature or after 14 days when kept refrigerated. Only clear solutions should be administered.
HOW SUPPLIED
TELAZOL (tiletamine HCl and zolazepam HCl) is available in individual vials of 5 mL solution when reconstituted. The addition of 5 mL diluent produces a solution containing the equivalent of 50 mg tiletamine base, 50 mg zolazepam base and 57.7 mg manitol per milliliter. 5 mL vial — 100 mg/mL (when reconstituted). Store at controlled room temperature 20° to 25°C (68° to 77°F).
Distributed by:
Zoetis Inc.
Kalamazoo, MI 49007
Made In Spain
Revised: June 2013
P625-526US/12-12
PRINCIPAL DISPLAY PANEL - 5 mL Bottle Carton
CIII
Telazol®
(TILETAMINE HCl
and ZOLAZEPAM HCl)
For Intramuscular Use
in Dogs and Cats Only
100 mg/mL
(when reconstituted)
CAUTION: Federal law restricts this drug
to use by or on the order of a licensed
veterinarian.
5 mL
NADA 106-111, Approved by FDA
zoetis
Telazoltiletamine hydrochloride and zolazepam hydrochloride INJECTION, POWDER, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||