Terazosin Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- TERAZOSIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- TERAZOSIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- TERAZOSIN HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
TERAZOSIN HYDROCHLORIDE DESCRIPTION

CLINICAL PHARMACOLOGY
PharmacodynamicsA. Benign Prostatic Hyperplasia (BPH)
**
Study 1Figure 1
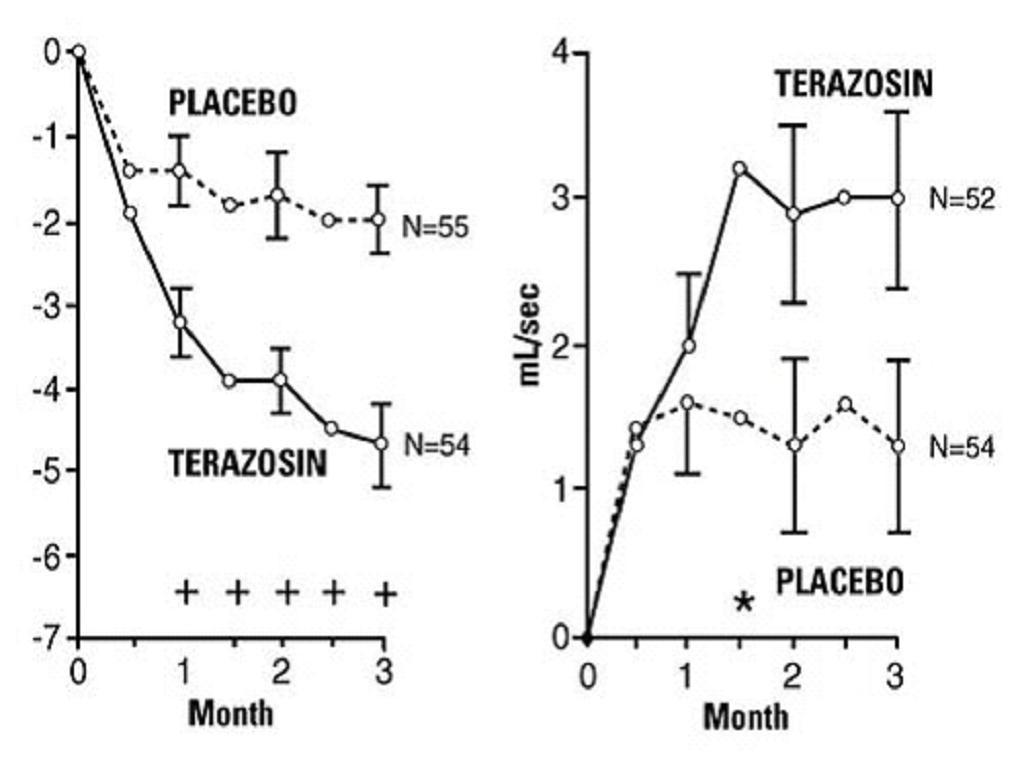
23
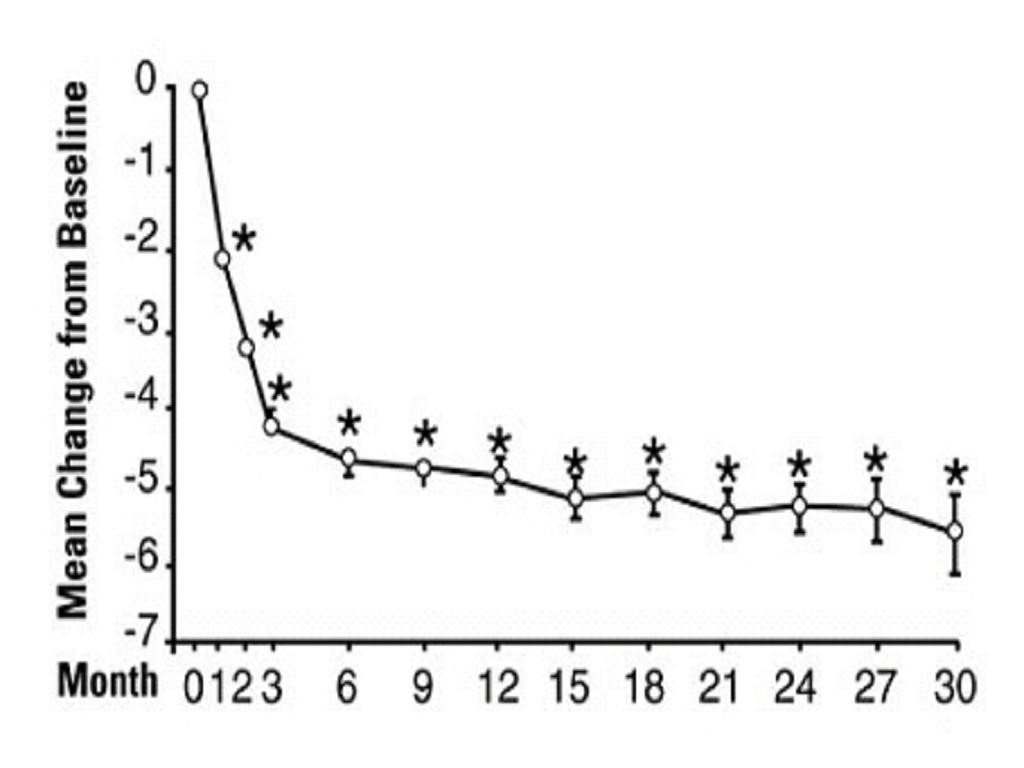
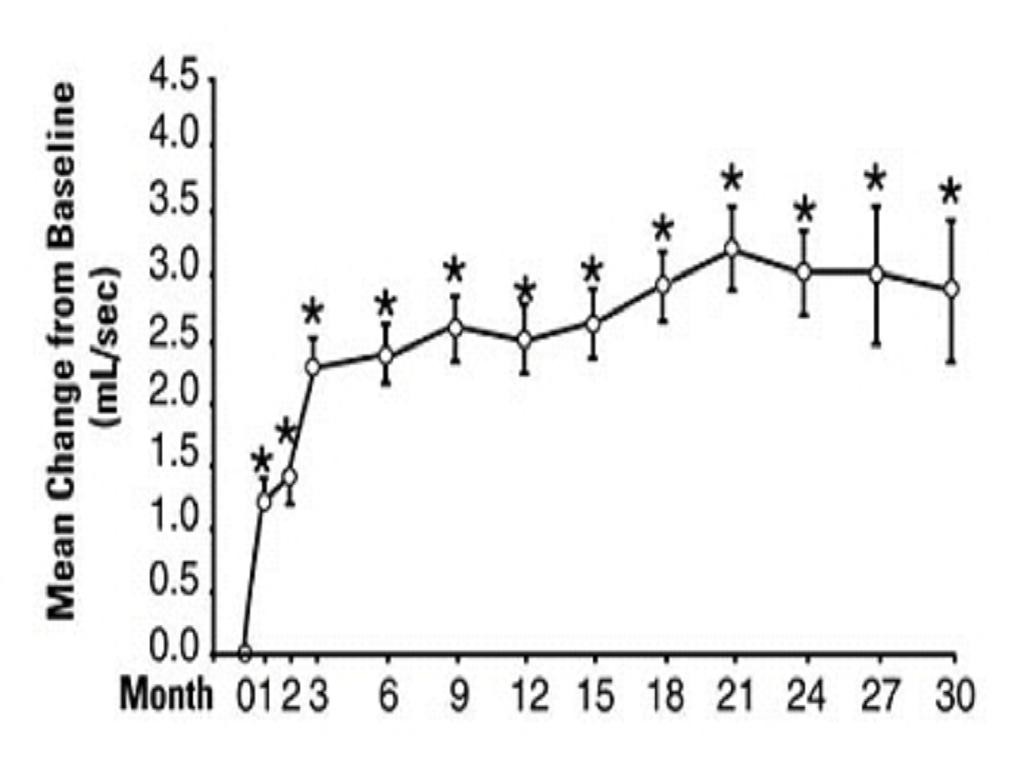
*****B. Hypertension
Pharmacokinetics
INDICATIONS & USAGE
TERAZOSIN HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Syncope andFirst-doseEffectTerazosin hydrochloride capsules, like other alpha-adrenergic blocking agents, can cause marked lowering of blood pressure, especially postural hypotension, and syncope in association with the first dose or first few days of therapy. A similar effect can be anticipated if therapy is interrupted for several days and then restarted. Syncope has also been reported with other alpha-adrenergic blocking agents in association with rapid dosage increases or the introduction of another antihypertensive drug. Syncope is believed to be due to an excessive postural hypotensive effect, although occasionally the syncopal episode has been preceded by about of severe supraventricular tachycardia with heart rates of120-160 beats per minute. Additionally, the possibility of the contribution of hemodilution to the symptoms of postural hypotension should be considered.
To decrease the likelihood of syncope or excessive hypotension, treatment should always be initiated witha1 mg dose of terazosin, given at bedtime. The 2mg, 5mg and 10 mg capsules are not indicated as initial therapy. Dosage should then be increased slowly, according to recommendations in the Dosage and Administration section and additional antihypertensive agents should be added with caution. The patient should be cautioned to avoid situations, such as driving or hazardous tasks, where injury could result should syncope occur during initiation of therapy.
CLINICAL PHARMACOLOGY
If syncope occurs, the patient should be placed in a recumbent position and treated supportively as necessary. There is evidence that the orthostatic effect of terazosin is greater, even in chronic use, shortly after dosing. The risk of the events is greatest during the initial seven days of treatment, but continues at all time intervals.
PRECAUTIONS
PRECAUTIONS
GeneralProstatic Cancer
Intraoperative Floppy Iris Syndrome (IFIS)
Orthostatic Hypotension
WARNINGS
INFORMATION FOR PATIENTS
(see Patient Package Insert):LABORATORY TESTS
DRUG INTERACTIONS
Use with Other Drugs:
DOSAGE AND ADMINISTRATION
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic effectsNonteratogenic effects
NURSING MOTHERS
PEDIATRIC USE
TERAZOSIN HYDROCHLORIDE ADVERSE REACTIONS
Benign Prostatic HyperplasiaTable 1PRECAUTIONS
**
Table 2
Hypertension
Table 3
**
Table 4
Post-marketing Experience
PRECAUTIONS
OVERDOSAGE
DOSAGE & ADMINISTRATION
Benign Prostatic Hyperplasia:
Initial Dose:
Subsequent Doses:
If terazosin administration is discontinued for several days or longer, therapy should be reinstituted using the initial dosing regimen.
Use with Other Drugs:
Precautions
Hypertension:
Initial Dose:
Subsequent Doses:
. If terazosin administration is discontinued for several days or longer, therapy should be reinstituted using the initial dosing regimen.
Use with other Drugs: (see above).
HOW SUPPLIED
STORAGE AND HANDLING
INFORMATION FOR PATIENTS
PATIENT INFORMATION ABOUTTERAZOSIN HYDROCHLORIDE
CAPSULES
When used to treat Hypertension or Benign Prostatic Hyperplasia (BPH)
What is hypertension (high blood pressure)?
Treatment options for hypertension
What TERAZOSIN HYDROCHLORIDE CAPSULES do to treat hypertension
What is BPH?
-
● a weak or interrupted stream when urinating
-
● a feeling that you can not empty your bladder completely
-
● a feeling of delay when you start to urinate
-
● a need to urinate often, especially at night, or
-
● a feeling that you must urinate right away
-
● Program of monitoring or "Watchful Waiting".
-
● Medication.There are different kinds of medication used to treat BPH. Your doctor has prescribed TERAZOSIN HYDROCHLORIDE CAPSULES for you. See "What TERAZOSIN HYDROCHLORIDE CAPSULES do to treat BPH" below.
-
● Surgery.Some patients may need surgery.
What TERAZOSIN HYDROCHLORIDE CAPSULES do to treat BPH
-
● TERAZOSIN HYDROCHLORIDE CAPSULES help relieve the symptoms of BPH. It does NOT change the size of the prostate, which may continue to grow. However, a larger prostate does not necessarily cause more or worse symptoms.
-
● If TERAZOSIN HYDROCHLORIDE CAPSULES are helping you, you should notice an effect on your particular symptoms in 2 to 4 weeks of starting to take the medication.
-
● Even though you take TERAZOSIN HYDROCHLORIDE CAPSULES and they may help you, TERAZOSIN HYDROCHLORIDE CAPSULES may not prevent the need for surgery in the future.
-
● You should see an effect on your symptoms in 2 to 4 week. So, you will need to continue seeing your doctor to check your progress regarding your BPH and to monitor your blood pressure in addition to your other regular check-ups.
-
● Your doctor has prescribed TERAZOSIN HYDROCHLORIDE CAPSULES for your BPH and not for prostate cancer. However, a man can have BPH and prostate cancer at the same time. Doctors usually recommend that men be checked for prostate cancer once a year when they turn 50 (or 40 if a family member has had prostate cancer). These checks should continue even if you are taking TERAZOSIN HYDROCHLORIDE CAPSULES. TERAZOSIN HYDROCHLORIDE CAPSULES are not a treatment for prostate cancer.
-
● About Prostate Specific Antigen (PSA). Your doctor may have done a blood test called PSA. Your doctor is aware that TERAZOSIN HYDROCHLORIDE CAPSULES do not affect PSA levels. You may want to ask your doctor more about this if you have had a PSA test done.
WARNINGS
TERAZOSIN HYDROCHLORIDE CAPSULES Can Cause A Sudden Drop In Blood Pressure After the VERY FIRST DOSE.
-
● You will start with a 1 mg dose of TERAZOSIN HYDROCHLORIDE CAPSULES. Then the dose will be increased as your body gets used to the effect of the medication.
-
● Other side effects you could have while taking TERAZOSIN HYDROCHLORIDE CAPSULES include drowsiness, blurred or hazy vision, nausea or "puffiness" of the feet or hands. Discuss any unexpected effects you notice with your doctor. Extremely rarely, TERAZOSIN HYDROCHLORIDE CAPSULES and similar medications have caused painful erection of the penis sustained for hours and unrelieved by sexual intercourse or masturbation. This condition is serious, and if untreated it can be followed by permanent inability to have an erection. If you have a prolonged abnormal erection, call your doctor or go to an emergency room as soon as possible.
FOR MORE INFORMATION ABOUT TERAZOSIN HYDROCHLORIDE CAPSULES AND HYPERTENSION OR BPH, TALK WITH YOUR DOCTOR, NURSE, PHARMACIST OR OTHER HEALTH CARE PROVIDER.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Terazosin HydrochlorideTerazosin Hydrochloride CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!