TERSI
Quinnova Pharmaceuticals, Inc.
TERSI
FULL PRESCRIBING INFORMATION: CONTENTS*
- TERSI DESCRIPTION
- CLINICAL PHARMACOLOGY
- TERSI INDICATIONS AND USAGE
- TERSI CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TERSI ADVERSE REACTIONS
- TERSI DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Can Label
- Folding Carton
FULL PRESCRIBING INFORMATION
TERSI Hydrating Topical Foam
(selenium sulfide in a water and lipid based foam, 2.25%)
Rx Only
TERSI DESCRIPTION
Selenium sulfide has the following chemical structure:
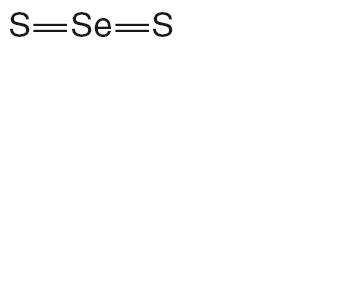
CLINICAL PHARMACOLOGY
TERSI INDICATIONS AND USAGE
TERSI CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
KEEP THIS AND ALL OTHER MEDICATIONS OUT OF THE REACH OF CHILDREN.
TERSI ADVERSE REACTIONS
TERSI DOSAGE AND ADMINISTRATION
TERSI should be shaken vigorously before each application and inverted to administer.

HOW SUPPLIED
TERSI is supplied in a 70 gram or 2.5 ounce aerosolized canister bearing the NDC Number 23710-225-70 and a 10 gram or 0.36 ounce aerosolized canister bearing the NDC Number 23710-025-01. The 10 gram canister is physician dispensed sample product.
Store at controlled room temperature 15º - 25ºC (59º - 77ºF).
Contains flammable materials. Contents under pressure. Do not puncture or incinerate. Do not expose to temperatures over 120ºF [48ºC] even when empty. Keep out of reach of children.
TERSI is covered by U.S. Patent 5,993,830.
TERSI is manufactured for Quinnova Pharmaceuticals, Inc., Newtown, PA 18940, (877) 6606263, www.QUINNOVA.com.
Prescribing Information as of September2007.

TRSFO025 7/08
Can Label

Folding Carton

TERSIselenium sulfide AEROSOL, FOAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||