TERUFLEX Blood Bag System Anticoagulant Citrate Phosphate Dextrose Adenine (CPDA-1)
TERUFLEX® BLOOD BAG SYSTEM with Blood Sampling Arm® CPDA-1 SOLUTION For the collection of 450 mL and 500 mL of Whole Blood
FULL PRESCRIBING INFORMATION
Uses
TERUFLEX® BLOOD BAG SYSTEM with Blood Sampling Arm ®
CPDA-1 SOLUTION
For the collection of 450 mL and 500 mL of Whole Blood
FULL PRESCRIBING INFORMATION
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1. To open blister package, peel cover film back 4/5 of its length.
2.2. Prepare the blood bag following your institution's standard operating procedures.
Evacuated blood collection tubes (glass or plastic)
2.3. Make a loose knot in the donor tubing below the "Y" unless alternate methods are used to seal the tubing at the end of collection.
2.4. Temporarily clamp donor tubing between the phlebotomy needle and the "Y".
2.5. Suspend the collection bag as far as possible below the donor's arm.
2.6. Apply blood pressure cuff or tourniquet to donor's arm. Disinfect site of phlebotomy. If blood pressure cuff is used, inflate to approximately 60 mmHg.
2.7. Remove the needle cover and perform phlebotomy.
2.7.1. CAUTION: Do not touch the needle after removing the needle cover.
2.8. Remove the temporary clamp on the donor tubing to permit blood flow into the collection bag.
2.9. If applicable, secure the needle safety device in place following the device instructions provided on the reverse side.
2.10. Secure donor tubing to donor's arm.
2.11. Mix blood with anticoagulant in the collection bag and continue to mix at several intervals during collection and immediately after collection. If using an automated mixer, follow manufacturer's instructions.
2.12. Collect labeled volume of blood 450 mL +/- 10% or 500 mL +/- 10%.
2.13. When the desired amount of blood has been collected, seal the tubing or tighten the loose knot (white knot) prepared in Step 2.3. Make a second seal between the first seal or knot and the "Y". Various methods may be used to seal tubing.
2.13.1. CAUTION: Do not use a dielectric tube sealer to seal the tubing while the needle is connected to the donor's body unless it is approved for such a purpose.
2.14. Anytime before Step # 2.19 below, sever donor tubing between two seals.
2.15. Assemble the luer adapter and the tube holder. These steps may be performed before or during phlebotomy.
2.15.1. Connect VENOJECT II Multi-Sample Luer Adapter to VENOJECT II Tube Holder (or equivalent) (Fig. 1).
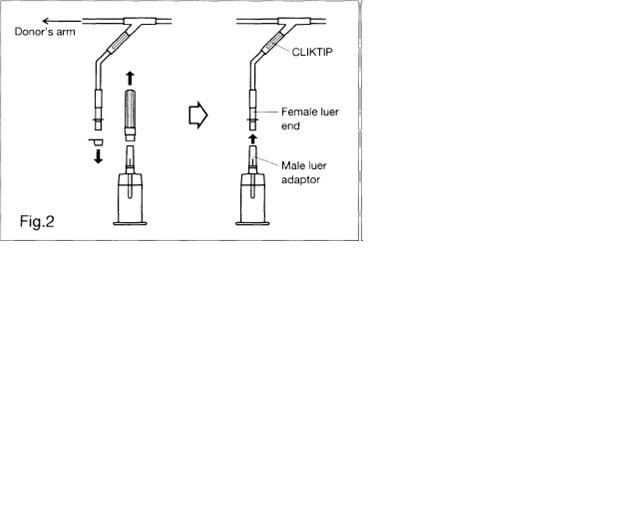
2.15.2. Remove covers and connect multi-sample luer adapter to female luer at end of Blood Sampling Arm (Fig. 2).

CAUTION:
CAUTION:
ooo
o
o
o
o
3. DOSAGE FORMS AND STRENGTHS
3.1. 63 mL Citrate Phosphate Dextrose Adenine (CPDA-1) anticoagulant USP for collection of 450 mL Whole Blood. Each 63 mL contains 188 mg Citric Acid (anhydrous) USP, 1.66 g Sodium Citrate (dihydrate) USP, 140 mg Monobasic Sodium Phosphate (monohydrate) USP, 2.01 g Dextrose (monohydrate) USP and 17.3 mg Adenine USP.
5. WARNINGS AND PRECAUTIONS
5.1. Rx ONLY.
5.2. Do not use unless solutions are clear and free from particulates.
5.3. Always inspect the blood bag set for leaks before use.
5.4. Avoid excessive heat and direct sunlight. Protect from freezing.
5.5. Recommended storage conditions: Room Temperature (15-30oC/59-86oF).
5.6. It is normal to have condensation in the blister packaging. If the amount of moisture is greater than expected, check for leaks from the fluid-filled components of the blood bag set.
5.7. Use aseptic techniques.
5.8. Do not use a dielectric tube sealer to seal the tubing while the needle is connected to the donor's body unless it is approved for such a purpose.
5.9. Do not touch needle after removing the needle cover.
5.10. Do no break CLIKTIP until luer adapter/tube holder assembly is attached to the Blood Sampling Arm.
5.11. Discard phlebotomy needle/donor tubing according to institutional procedures.
5.12. The AGELESS (oxygen absorber packet, Mitsubishi Gas Chemical) contained in this package absorbs oxygen and generates heat on removal. Do not open and handle it with care.
5.13. Dispose of the AGELESS packet with the blister tray.
5.14. Do not dispose the AGELESS packet with wastes containing volatile or flammable materials.
5.15. Due to possible exposure to infectious agents in the handling of blood, take adequate precautions at all times to prevent exposure to and transmission of such agents. Follow you institution's standard operating procedures.
11. DESCRIPTION/PRODUCT SPECIFICATIONS
11.1. This blood bag system includes a 16 gauge x 1 1/2 inch (1.60 x 38 mm) needle with needle cover and either a 450 mL or 500 mL (nominal capacity 600 mL) primary collection bag containing 63 mL or 70 mL, respectively, Citrate Phosphate Dextrose Adenine (CPDA-1) anticoagulant. The Double blood bag set has one integrally attached empty satellite bag (nominal capacity 400 mL). The Triple blood bag set has one integrally attached empty satellite bag (nominal capacity 400 mL) and one empty XT-612 5 day Platelet bag (nominal capacity 500 mL). The Quadruple blood bag set has two integrally attached empty satellite bags (nominal capacity 400 mL) and one empty XT-612 5 day Platelet bag (nominal capacity 500 mL).
11.2. Blood bag codes ending in A4 are supplied with Integral Blood Sampling Arm for obtaining donor samples for laboratory testing after collection of the Whole Blood unit.
11.3. Blood bag codes ending in A3 also include a DonorCare Needle Guard pre-attached to the donor tubing. DonorCare Needle Guard device instructions are provided on the reverse side.
11.4. The blood bag collection set is made of PVC (polyvinyl chloride with DEHP plasticizer).
11.5. The blood bag has no components made of natural rubber latex.
11.6. Tubing internal diameter (ID) nominal 3.0 mm.
11.7. Tubing outer diameter (OD) nominal 4.4 mm.
11.8. Donor tubing line maximum 16 segments available.
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1. Single use only.
16.2. Sterile and non-pyrogenic fluid path. Sterilized by steam. Opacity of the blood bag system may be observed. This is due to moisture absorption during the sterilization process. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
16.3. A Material Safety Data Sheet (MSDS) is not required for this product.
16.4. Recommended storage conditions: Room Temperature (15-30oC/59-86oF).
16.5. Avoid excessive heat and direct sunlight. Protect from freezing.
16.6. To open blister package, peel cover film back 4/5 of its length.
Figure Sealing with tape

16.7. After opening the blister package, unused blood bags may be stored at room temperature for 96 hours or they may be stored for 30 days by returning cover film to original position and sealing with tape to prevent evaporation of solutions.
16.8. Blood bags in the unopened blister package may be used through the last day of the month and year as indicated on the original manufacturer's packaging.
16.9. The AGELESS packet contained in this package absorbs oxygen and generates heat on removal. Do not open and handle it with care.
16.10. Dispose of the AGELESS packet with the blister tray.
16.11. Do not dispose the AGELESS packet with wastes containing volatile or flammable materials.
16.12. For the Single blood bag sets, Codes BB*SCD456A3 and BB*SCD506A3 are supplied 36/case, Code BB*SCD456A4 is supplied 48/case.
16.13. For the Double blood bag sets, Codes BB*DCD456A3, BB*DCD456A4 and BB*DCD506A3 are supplied 30/case.
16.14. For the Triple blood bag sets, Codes BB*TCD456A3 and BB*TCD456A4 are supplied 30/case.
16.15. For the Quadruple blood bag sets, Codes BB*QCD456A3 and BB*QCD506A3 are supplied 24/case.
MANUFACTURED BY:
TERUMO CORPORATION
44-1, 2-CHOME, HATAGAYA, SHIBUYA-KU, TOKYO 151-0072, JAPAN
MADE IN JAPAN
(R): Registered Trademark
(c) TERUMO CORPORATION November, 2011 11K09
DonorCare is a registered trademark of ITL Corporation, Melbourne, Australia.
AGELESS is a registered trademark of MITSUBISHI GAS CHEMICAL CO., INC.
Tray/Case Label
TERUFLEX® BLOOD BAG SYSTEM with Blood Sampling ArmTM
ANTICOAGULANT CITRATE PHOSPHATE
DEXTROSE ADENINE SOLUTION (CPDA-1) USP
FOR COLLECTION OF 500mL OF BLOOD
Each unit consists of a primary bag containing 70 mL of solution with
209 mg Citric Acid (anhydrous) USP, 1.84 9 Sodium Citrate (dihydrate)
USP, 155 mg Monobasic Sodium Phosphate (monohydrate) USP, 2.23 9
Dextrose (monohydrate) USP, 19.3 mg Adenine USP.
STERILE, NON-PYROGENIC FLUID PATH.
DO NOT USE UNLESS ANTICOAGULANT IS CLEAR.
CODE
LOT NO.
EXPIRY
UNITS
DONOR NEEDLE
16G X 1 1/2" (1.60 X 38mm)
RxONLY
RECOMMENDED STORAGE: Room Temperature (15-30°C/59-86°F).
Avoid excessive heat. Protect from freezing.
After opening, unused bags may be stored for 30 days by returning cover film to original
position and sealing with tape to prevent possible loss of moisture.
See Instructions For Blood Collection.
Manufactured by: TERUMO CORPORATION Tokyo, Japan
®: Registered Trademark Blood Sampling Arm is a trademark of TERUMO CORPORATION.
Rev. 01/03
B-2-H6-A4-2

TERUFLEX Blood Bag System Anticoagulant Citrate Phosphate Dextrose Adenine (CPDA-1)Anticoagulant Citrate Phosphate Dextrose Adenine (CPDA-1) SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||