Tetrofosmin
Tetrofosmin (for the preparation of Tc99m Tetrofosmin injection)
FULL PRESCRIBING INFORMATION
Dear Medical Professional,
Per your order, we have compounded Tetrofosmin as a sterile, freeze-dried preparation in a 10 mL vial. The characteristics of this preparation are described below.
AnazaoHealth’s compounded Tetrofosmin vial is a sterile, non-pyrogenic preparation that consists of a lyophilized mixture of 0.35 mg of Tetrofosmin, 1.5 mg of D-Gluconate, 0.03 mg of Stannous Chloride Dihydrate, 0.48 mg of Disodium Sulphosalicylate, and 2.7 mg of Sodium Hydrogen Carbonate and is maintained under an inert nitrogen atmosphere. It contains no antimicrobrial preservative.
Tetrofosmin is a diagnostic agent used to assess areas of reversible myocardial ischemia in the presence or absence of infracted myocardium and is also used to assess ventricular function.
PHYSICAL HALF-LIFE & TARGET ORGANS
The physical half-life of technetium, Tc99m, is 6 hours and has a principal radiation emission of gamma photons with a mean energy of 140 KeV.
| Absorbed radiation dose | ||||
| Exercise | Rest | |||
| Target organ | rad/mCi | µGy/MBq | rad/mCi | µGy/MBq |
| Gall bladder wall | 0.123 | 33.2 | 0.180 | 48.6 |
| Upper large intestine | 0.075 | 20.1 | 0.113 | 30.4 |
| Bladder wall | 0.058 | 15.6 | 0.071 | 19.3 |
| Lower large intestine | 0.057 | 15.3 | 0.082 | 22.2 |
| Small intestine | 0.045 | 12.1 | 0.063 | 17.0 |
| Kidney | 0.039 | 10.4 | 0.046 | 12.5 |
| Salivary glands | 0.030 | 8.04 | 0.043 | 11.6 |
| Ovaries | 0.029 | 7.88 | 0.035 | 9.55 |
| Uterus | 0.027 | 7.34 | 0.031 | 8.36 |
| Bone surface | 0.023 | 6.23 | 0.021 | 5.58 |
| Pancreas | 0.019 | 5.00 | 0.018 | 4.98 |
| Stomach | 0.017 | 4.60 | 0.017 | 4.63 |
| Thyroid | 0.016 | 4.34 | 0.022 | 5.83 |
| Adrenals | 0.016 | 4.32 | 0.015 | 4.11 |
| Heart wall | 0.015 | 4.14 | 0.015 | 3.93 |
| Red marrow | 0.015 | 4.14 | 0.015 | 3.97 |
| Spleen | 0.015 | 4.12 | 0.014 | 3.82 |
| Muscle | 0.013 | 3.52 | 0.012 | 3.32 |
| Testes | 0.013 | 3.41 | 0.011 | 3.05 |
| Liver | 0.012 | 3.22 | 0.015 | 4.15 |
| Thymus | 0.012 | 3.11 | 0.009 | 2.54 |
| Brain | 0.010 | 2.72 | 0.008 | 2.15 |
| Lungs | 0.008 | 2.27 | 0.008 | 2.08 |
| Skin | 0.008 | 2.22 | 0.007 | 1.91 |
| Breasts | 0.008 | 2.22 | 0.007 | 1.83 |
Dose calculations were performed using the standard MIRD method (MIRD Pamphlet No.1 (rev),Society of Nuclear Medicine, 1976).
Effective dose equivalents (EDE) were calculated in accordance with ICRP 53 (Ann. ICRP 18 (1-4),1988) and gave values of 8.61 × 10-3 mSV/MBq and 1.12 × 10-2 mSV/MBq after exercise and rest, respectively.
The structural formula for tetrofosmin is:
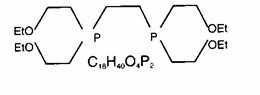
When Tetrofosmin is reconstituted with Tc99m pertechnetate, a complex of Tc99m Tetrofosmin is formed and is the active ingredient of the reconstituted product. When administered intravenously, Tc99m Tetrofosmin shows rapid myocardial uptake and its distribution follows a linear relationship with coronary blood flow.
Tc99m Tetrofosmin is a lipophilic agent that is taken up by the mitochondria of myocardial cells by passive diffusion and appears to accumulate in viable myocardial tissue.
There are no known contraindications for this preparation.
Depending on the protocol for rest/stress imaging, doses of 10 to 30 mCi (370 to 1110 MBq) are given intravenously
PREPARATION
- Snap off the plastic lid and place in appropriate lead shielding. Wipe the septum with 70% isopropyl alcohol and allow it to dry.
- Using a 10 mL syringe, dilute up to 360 mCi of Tc99m with saline and add to vial. The total volume should be between 6 mL -12 mL and the Tc99m concentration should not exceed 30 mCi/mL (360 mCi/12 mL).
- After adding the Tc99m, insert a 0.22 µm filtered vent needle and, using a separate syringe, withdraw 3 mL of gas from the vial, allowing sterile filtered air into the vial. Remove vent needle and syringe.
- Mix gently and invert several times for 10 seconds.
- Let stand for 15 minutes at room temperature as the complexes form.
- Inspect vial through a lead glass shield for particulate matter. Do not use if the solution is not clear.
- Store at 2°- 8°C (36°- 46°F) and use within 12 hours after mixing. Radiochemical purity should be at least 90% prior to administration
- QC info: Solvent – Ethyl Acetate, Strip – Whatman CHR% Tag = top counts/total counts X 100%
STORAGE
The preparation should be stored in the refrigerator at 2- 8(C (36 - 46(F) and protected from light.

TetrofosminTetrofosmin INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||