TEV-TROPIN
Gate Pharmaceuticals
Ferring Pharmaceuticals Inc.
TEV-TROPIN [somatropin (rDNA origin) for injection]5 mg and 10 mg
FULL PRESCRIBING INFORMATION: CONTENTS*
- TEV-TROPIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATION AND USAGE
- TEV-TROPIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TEV-TROPIN ADVERSE REACTIONS
- OVERDOSAGE
- TEV-TROPIN DOSAGE AND ADMINISTRATION
- STABILITY AND STORAGE
- HOW SUPPLIED
- PATIENT MEDICATION INFORMATION
- PACKAGE LABEL - TEV-TROPIN 5mg LABEL
- PACKAGE LABEL - DILUENT 5mL LABEL
- PACKAGE LABEL - TEV-TROPIN 5mg CARTON
- PACKAGE LABEL - TEV-TROPIN 10mg LABEL
- PACKAGE LABEL - DILUENT 1mL LABEL
- PACKAGE LABEL - 10 mg CARTON
FULL PRESCRIBING INFORMATION
TEV-TROPIN DESCRIPTION
TEV-TROPIN® (somatropin, rDNA origin, for injection), a polypeptide of recombinant DNA origin, has 191 amino acid residues and a molecular weight of about 22,124 daltons. It has an amino acid sequence identical to that of human growth hormone of pituitary origin. TEV-TROPIN® is a strain of Escherichia coli modified by insertion of the human growth hormone gene.
TEV-TROPIN® is a sterile, white, lyophilized powder, intended for subcutaneous administration, after reconstitution with the accompanying diluent.
TEV-TROPIN® 5 mg vial contains recombinant somatropin 5 mg and mannitol 30 mg. The 5 mg vial is supplied in a combination package with an accompanying 5 mL vial of diluting solution. The diluent contains bacteriostatic 0.9% sodium chloride injection, USP, (normal saline), 0.9% benzyl alcohol as a preservative, and water for injection.
TEV-TROPIN® 10 mg vial contains recombinant somatropin 10 mg, mannitol 10 mg, disodium phosphate dodecahydrate 3.57 mg, and sodium dihydrogen phosphate dehydrate 0.79 mg. The 10 mg vial is supplied in a combination package with an accompanying 1 mL syringe of diluting solution. The diluent contains bacteriostatic water for injection with 0.33% metacresol as a preservative.
TEV-TROPIN® is a highly-purified preparation. Reconstituted solutions have a pH in the range of 7.0 to 9.0.
CLINICAL PHARMACOLOGY
Clinical trials have demonstrated that TEV-TROPIN® is equivalent in its therapeutic effectiveness and in its pharmacokinetic profile to those of human growth hormone of pituitary origin (somatropin). TEV-TROPIN® stimulates linear growth in children who lack adequate levels of endogenous growth hormone. Treatment of growth hormone-deficient children with TEV-TROPIN® produces increased growth rates and IGF-1 (Insulin-Like Growth Factor-1) concentrations that are similar to those seen after therapy with human growth hormone of pituitary origin.
Both TEV-TROPIN® and somatropin have also been shown to have other actions including:
-
Tissue Growth
- Skeletal Growth. TEV-TROPIN® stimulates skeletal growth in patients with growth hormone deficiency. The measurable increase in body length after administration of TEV-TROPIN® results from its effect on the epiphyseal growth plates of long bones. Concentration of IGF-1, which may play a role in skeletal growth, are low in the serum of growth hormone-deficient children but increase during treatment with TEV-TROPIN®. Mean serum alkaline phosphatase concentrations are increased.
- Cell Growth. It has been shown that there are fewer skeletal muscle cells in short statured children who lack endogenous growth hormone as compared with normal children. Treatment with somatropin results in an increase in both the number and size of muscle cells.
- Organ Growth. Somatropin influences the size of internal organs and it also increases red cell mass.
-
Protein Metabolism
Linear growth is facilitated, in part, by increased cellular protein synthesis. Nitrogen retention, as demonstrated by decreased urinary nitrogen excretion and serum urea nitrogen, results from treatment with somatropin. -
Carbohydrate Metabolism
Children with hypopituitarism sometimes experience fasting hypoglycemia that is improved by treatment with somatropin. Large doses of somatropin may impair glucose tolerance. -
Lipid Metabolism
Administration of somatropin to growth hormone-deficient patients mobilizes lipid, reduces body fat stores, and increases plasma fatty acids. -
Mineral Metabolism
Sodium, potassium, and phosphorous are conserved by somatropin. Serum concentrations of inorganic phosphates increased in patients with growth hormone deficiency after therapy with TEV-TROPIN® or somatropin. Serum calcium concentrations are not significantly altered in patients treated with either somatropin or TEV-TROPIN®. -
Connective Tissue Metabolism
Somatropin stimulates the synthesis of chondroitin sulfate and collagen as well as the urinary excretion of hydroxyproline.
PHARMACOKINETICS
Following intravenous administration of 0.1 mg/kg of TEV-TROPIN®, the elimination half-life was about 0.42 hours (approximately 25 minutes) and the mean plasma clearance (±SD) was 133 (±16) mL/min in healthy male volunteers.
In the same volunteers, after a subcutaneous injection of 0.1 mg/kg TEV-TROPIN® to the forearm, the mean peak serum concentration (±SD) was 80 (±50) ng/mL which occurred approximately 7 hours post-injection and the apparent elimination half-life was approximately 2.7 hours. Compared to intravenous administration, the extent of systemic availability from subcutaneous administration was approximately 70%.
INDICATION AND USAGE
TEV-TROPIN® is indicated for the treatment of children who have growth failure due to an inadequate secretion of normal endogenous growth hormone.
TEV-TROPIN CONTRAINDICATIONS
TEV-TROPIN®
5 mg reconstituted with bacteriostatic 0.9% sodium chloride injection, USP (normal saline) (benzyl alcohol preserved) should not be administered to patients with a known sensitivity to benzyl alcohol (see WARNINGS).
TEV-TROPIN® 10 mg reconstituted with bacteriostatic water for injection containing 0.33% metacresol should not be used if the patient is allergic to metacresol.
Somatropin should not be used for growth promotion in pediatric patients with closed epiphyses.
Somatropin is contraindicated in patients with active proliferative or severe non-proliferative diabetic retinopathy.
In general, somatropin is contraindicated in the presence of active malignancy. Any preexisting malignancy should be inactive and its treatment complete prior to instituting therapy with somatropin. Somatropin should be discontinued if there is evidence of recurrent activity. Since growth hormone deficiency may be an early sign of the presence of a pituitary tumor (or, rarely, other brain tumors), the presence of such tumors should be ruled out prior to initiation of treatment. Somatropin should not be used in patients with any evidence of progression or recurrence of an underlying intracranial tumor.
Treatment with pharmacologic amounts of somatropin is contraindicated in patients with acute critical illness due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure. Two placebo-controlled clinical trials in non-growth hormone deficient adult patients (n = 522) with these conditions in intensive care units revealed a significant increase in mortality (41.9% vs. 19.3%) among somatropin-treated patients (doses 5.3 to 8 mg/day) compared to those receiving placebo (see WARNINGS).
Somatropin is contraindicated in patients with Prader-Willi syndrome who are severely obese or have severe respiratory impairment (see WARNINGS). TEV-TROPIN® is not indicated for the treatment of pediatric patients who have growth failure due to genetically confirmed Prader-Willi syndrome.
WARNINGS
Increased mortality in patients with acute critical illness due to complications following open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure has been reported after treatment with pharmacologic doses of somatropin (see CONTRAINDICATIONS ). The safety of continuing somatropin treatment in patients receiving replacement doses for approved indications who concurrently develop these illnesses has not been established. Therefore, the potential benefit of treatment continuation with somatropin in patients experiencing acute critical illnesses should be weighed against the potential risk.
There have been reports of fatalities after initiating therapy with somatropin in pediatric patients with Prader-Willi syndrome who had one or more of the following risk factors: severe obesity, history of upper airway obstructions or sleep apnea, or unidentified respiratory infection. Male patients with one or more of these factors may be at greater risk than females. Patients with Prader-Willi syndrome should be evaluated for signs of upper airway obstruction and sleep apnea before initiation of treatment with somatropin. If during treatment with somatropin, patients show signs of upper airway obstruction (including onset of or increased snoring) and/or new onset sleep apnea, treatment should be interrupted. All patients with Prader-Willi syndrome treated with somatropin should also have effective weight control and be monitored for signs of respiratory infection, which should be diagnosed as early as possible and treated aggressively (see CONTRAINDICATIONS ). TEV-TROPIN® is not indicated for the treatment of pediatric patients who have growth failure due to genetically confirmed Prader-Willi syndrome.
Cases of pancreatitis have been reported rarely in children and adults receiving somatropin treatment, with some evidence supporting a greater risk in children compared with adults. Published literature indicates that girls who have Turner syndrome may be at greater risk than other somatropin-treated children. Pancreatitis should be considered in any somatropin-treated patient, especially a child, who develops persistent, severe abdominal pain.
Benzyl alcohol, a component used to reconstitute the TEV-TROPIN® 5 mg vial, has been associated with serious adverse events and death, particularly in pediatric patients. The “gasping syndrome,” (characterized by central nervous system depression, metabolic acidosis, gasping respirations, and high levels of benzyl alcohol and its metabolites found in the blood and urine) has been associated with benzyl alcohol dosages >99 mg/kg/day in neonates and low-birth weight neonates. Additional symptoms may include gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Practitioners administering this and other medications containing benzyl alcohol should consider the combined daily metabolic load of benzyl alcohol from all sources.
When administering TEV-TROPIN® 5 mg to newborns, reconstitute with sterile normal saline for injection, USP. WHEN RECONSTITUTING WITH STERILE NORMAL SALINE, USE ONLY ONE DOSE PER VIAL AND DISCARD THE UNUSED PORTION.
PRECAUTIONS
General
TEV-TROPIN® therapy should be carried out under the regular guidance of a physician who is experienced in the diagnosis and management of pediatric patients with growth hormone deficiency.
Patients with preexisting tumors or growth hormone deficiency secondary to an intracranial lesion should be examined routinely for progression or recurrence of the underlying disease process. In pediatric patients, clinical literature has not revealed a relationship between somatropin replacement therapy and recurrence of CNS tumors. However, in childhood cancer survivors, an increased risk of a second neoplasm has been reported in patients treated with somatropin after their first neoplasm. Intracranial tumors, in particular meningiomas, in patients treated with radiation to the head for their first neoplasm, were the most common of these second neoplasms. In adults, it is unknown whether there is any relationship between somatropin replacement therapy and CNS tumor recurrence.
Patients should be monitored carefully for any malignant transformation of skin lesions.
Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses in susceptible patients. As a result, previously undiagnosed impaired glucose tolerance and overt diabetes mellitus may be unmasked during somatropin treatment. New-onset type 2 diabetes mellitus has been reported in patients. Therefore, glucose levels should be monitored periodically in all patients treated with somatropin, especially in those with risk factors for diabetes mellitus, such as obesity, Turner syndrome, or a family history of diabetes mellitus. Patients with preexisting type 1 or type 2 diabetes mellitus or impaired glucose tolerance should be monitored closely during somatropin therapy. The doses of antihyperglycemic drugs (i.e., insulin or oral agents) may require adjustment when somatropin therapy is instituted in these patients.
In patients with hypopituitarism (multiple hormone deficiencies), standard hormonal replacement therapy should be monitored closely when somatropin therapy is administered. Undiagnosed/untreated hypothyroidism may prevent an optimal response to somatropin, in particular, the growth response in children. Patients with Turner syndrome have an inherently increased risk of developing autoimmune thyroid disease and primary hypothyroidism. In patients with growth hormone deficiency, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Therefore, patients treated with somatropin should have periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or appropriately adjusted when indicated.
Patients with endocrine disorders, including growth hormone deficiency, may have an increased incidence of slipped capital femoral epiphysis. Any child who develops a limp or complains of hip or knee pain during somatropin therapy should be evaluated.
Intracranial hypertension (IH) with papilledema, visual changes, headache, nausea and/or vomiting has been reported in a small number of patients treated with growth hormone products. IH has been reported more frequently after treatment with IGF-1. Symptoms usually occur within the first eight weeks after the initiation of growth hormone therapy. In all reported cases, IH-associated signs and symptoms resolved rapidly after temporary suspension or termination of therapy. Funduscopic examination should be performed routinely before initiating treatment with somatropin to exclude preexisting papilledema and periodically during the course of somatropin therapy. If papilledema is observed by funduscopy during somatropin treatment, treatment should be stopped. If somatropin induced idiopathic IH is diagnosed, treatment with somatropin can be restarted at a lower dose after IH-associated signs and symptoms have resolved.
Progression of scoliosis can occur in children who experience rapid growth. Because somatropin increases growth rate, patients with a history of scoliosis who are treated with somatropin should be monitored for progression of scoliosis.
Bone age should be monitored periodically during somatropin administration, especially in patients who are pubertal and/or receiving concomitant thyroid hormone replacement therapy. Under these circumstances, epiphyseal maturation may progress rapidly.
When somatropin is administered subcutaneously at the same site over a long period of time, tissue atrophy may result. This can be avoided by rotating the injection site. As is the case with any protein, local or systemic allergic reactions may occur. Parents/Patient should be informed that such reactions are possible and that prompt medical attention should be sought if allergic reactions occur.
Information for Patients
Patients being treated with TEV-TROPIN® and/or their caregivers should be informed about the potential benefits and risks associated with treatment. See the patient information included with the product and/or injection device. This information is intended to aid in the safe and effective administration of the medication. It is not a disclosure of all possible adverse or intended effects.
Patients and caregivers who will administer TEV-TROPIN® should receive appropriate training and instruction on the proper use of TEV-TROPIN® from the physician or other suitable qualified health care professional. A puncture-resistant container for the disposal of used needles and syringes should be strongly recommended. Patients and/or caregivers should be thoroughly instructed in the importance of proper disposal, and cautioned against any reuse of needles and syringes.
Laboratory Tests
Serum levels of inorganic phosphorus, alkaline phosphatase, and IGF-1 may increase after somatropin therapy.
Drug Interactions
The microsomal enzyme 11β-hydroxysteroid dehydrogenase type 1 (11βHSD-1) is required for conversion of cortisone to its active metabolite, cortisol, in hepatic and adipose tissue. Growth hormone and somatropin inhibit 11βHSD-1. Consequently, individuals with untreated GH deficiency have relative increases in 11βHSD-1 and serum cortisol. Introduction of somatropin treatment may result in inhibition of 11βHSD-1 and reduced serum cortisol concentrations. As a consequence, previously undiagnosed central (secondary) hypoadrenalism may be unmasked and glucocorticoid replacement may be required in patients treated with somatropin. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of somatropin treatment; this may be especially true for patients treated with cortisone acetate and prednisone since conversion of these drugs to their biologically active metabolites is dependent on the activity of 11βHSD-1.
Pharmacologic glucocorticoid therapy and supraphysiologic glucocorticoid treatment may attenuate the growth promoting effects of somatropin in children. Therefore, glucocorticoid replacement dosing should be carefully adjusted in children receiving concomitant somatropin and glucocorticoid treatments to avoid both hypoadrenalism and an inhibitory effect on growth.
Limited published data indicate that somatropin treatment increases cytochrome P450 (CP450) mediated antipyrine clearance in man. These data suggest that somatropin administration may alter the clearance of compounds known to be metabolized by CP450 liver enzymes (e.g., corticosteroids, sex steroids, anticonvulsants, cyclosporine). Careful monitoring is advisable when somatropin is administered in combination with other drugs known to be metabolized by CP450 liver enzymes.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis, mutagenesis, and reproduction studies have not been conducted with TEV-TROPIN®.
Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with TEV-TROPIN®. It is also not known whether TEV-TROPIN® can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. TEV-TROPIN® should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when TEV-TROPIN® is administered to a nursing woman.
Geriatric Use
The safety and effectiveness of somatropin in patients aged 65 and over has not been evaluated in clinical studies. Elderly patients may be more sensitive to the action of somatropin, and may be more prone to develop adverse reactions.
TEV-TROPIN ADVERSE REACTIONS
The following adverse reactions have been observed during appropriate use of somatropin: headaches (children and adults), gynecomastia (children) and pancreatitis (children and adults) (see WARNINGS ).
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influences by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to TEV-TROPIN® with the incidence of antibodies to other products may be misleading. With respect to growth hormone, antibody binding capacities below 2 mg/L have not been associated with growth attenuation. In some cases, when binding capacity exceeds 2 mg/L, growth attenuation has been observed.
None of the patients with anti-GH antibodies in the clinical studies experienced decreased linear growth response to TEV-TROPIN® or any other associated adverse event. Injection site reactions (e.g., pain, bruise) occurred in 8 of the 164 treated patients.
Leukemia has been reported in a small number of patients treated with other growth hormone products. It is uncertain whether this risk is related to the pathology of growth hormone deficiency itself, growth hormone therapy, or other associated treatments such as radiation therapy for intracranial tumors.
New-onset type 2 diabetes mellitus has been reported.
OVERDOSAGE
The recommended dosage of up to 0.1 mg/kg of body weight 3 times per week should not be exceeded. Acute overdose could cause initial hypoglycemia and subsequent hyperglycemia. Repeated use of doses in excess of those recommended could result in signs and symptoms of gigantism and/or acromegaly consistent with the known effects of excess human growth hormone.
TEV-TROPIN DOSAGE AND ADMINISTRATION
A dosage of up to 0.1 mg/kg of body weight administered 3 times per week by subcutaneous injection is recommended.
TEV-TROPIN® 5 mg should be reconstituted with 1 to 5 mL of bacteriostatic 0.9% sodium chloride for injection, USP (benzyl alcohol preserved). Reconstituted TEV-TROPIN® 5 mg vials should not be used if the patient has a known sensitivity to benzyl alcohol. Benzyl alcohol as a preservative in bacteriostatic normal saline, USP, has been associated with toxicity in newborns. WHEN ADMINISTERING TEV-TROPIN® TO NEWBORNS, RECONSTITUTE WITH STERILE NORMAL SALINE FOR INJECTION, USP.
TEV-TROPIN® 10 mg should be reconstituted with 1 mL syringe of bacteriostatic water for injection containing 0.33% metacresol as a preservative. Reconstituted TEV-TROPIN® 10 mg vials should not be used if the patient is allergic to metacresol.
The stream of normal saline should be aimed against the side of the vial to prevent foaming. Swirl the vial with a GENTLE rotary motion until the contents are completely dissolved and the solution is clear. DO NOT SHAKE. Since TEV-TROPIN® is a protein, shaking or vigorous mixing will cause the solution to be cloudy. If the resulting solution is cloudy or contains particulate matter, the contents MUST NOT be injected.
Occasionally, after refrigeration, some cloudiness may occur. This is not unusual for proteins like TEV-TROPIN®. Allow the product to warm to room temperature. If cloudiness persists or particulate matter is noted, the contents MUST NOT be used.
Before and after injection, the septum of the vial should be wiped with rubbing alcohol or an alcoholic antiseptic solution to prevent contamination of the contents by repeated needle insertions.
TEV-TROPIN® 5 mg can be administered using a standard sterile disposable syringe or a Tjet Needle-Free injection device. However, TEV-TROPIN® 10 mg can only be administered using a standard sterile disposable syringe. For proper use, please refer to the User's Manual provided with the administration device.
STABILITY AND STORAGE
Before Reconstitution
Vials of TEV-TROPIN® (5 and 10 mg) are stable when refrigerated at 36° to 46°F (2° to 8°C). Avoid freezing the accompanying diluent. Expiration dates are stated on the labels.
After Reconstitution
TEV-TROPIN® 5 mg is stable for up to 14 days when reconstituted with bacteriostatic 0.9% sodium chloride (normal saline), USP, and stored in a refrigerator at 36° to 46°F (2° to 8°C). Do not freeze the reconstituted solution.
TEV-TROPIN® 10 mg is stable for up to 28 days when reconstituted with 1 mL syringe of bacteriostatic water for injection containing 0.33% metacresol as a preservative, and stored in a refrigerator at 36° to 46°F (2° to 8°C). Do not freeze the reconstituted solution.
HOW SUPPLIED
TEV-TROPIN® (somatropin, rDNA origin, for injection) is supplied as 5 mg and 10 mg of lyophilized, sterile somatropin per vial.
TEV-TROPIN® 5 mg carton contains one vial of TEV-TROPIN® (5 mg per vial) and one vial of diluent [5 mL of bacteriostatic 0.9% sodium chloride for injection, USP (benzyl alcohol preserved)], and is supplied in single cartons.
TEV-TROPIN® 10 mg carton contains one vial of TEV-TROPIN® (10 mg per vial), one syringe of diluent [1 mL of bacteriostatic water for injection with 0.33% metacresol as preservative] and a 25G reconstitution needle, and is supplied in single cartons.
Rx only.
Distributed By:Teva Select Brands
Horsham, PA 19044
Division of Teva Pharmaceuticals USA
Rev. M 03/2012
0082-5008v8
PATIENT MEDICATION INFORMATION
Do not mix (reconstitute) the drug or inject it until you have been thoroughly trained in the proper techniques by your doctor. Use sterile techniques as instructed by your doctor. The first injection of TEV-TROPIN should be given under the supervision of your doctor. Properly discard syringes and/or needles after each use.
PREPARATION
Your GROWTH HORMONE is supplied in a powder form that must be prepared for injection by mixing it with a diluent (a liquid, either Bacteriostatic 0.9% Sodium Chloride Injection, USP [5 mg vial] or Bacteriotstatic Water for Injection with 0.33% Metacresol [10 mg vial]) prior to use. Only use the specific
diluent
that is supplied in the carton containing your GROWTH HORMONE. Once you have reconstituted your GROWTH HORMONE with the diluent, it is ready for injection. Your doctor will prescribe the exact amount of diluent and tell you the size of the syringe and needle to use.
Note: The diluents are different for the 5 mg and 10 mg vials. Do not interchange the diluents.
TEV-TROPIN should be kept refrigerated (36° to 46°F [2°to 8°C]) before and after reconstitution. Do not freeze.
TEV-TROPIN 5 mg is supplied with a vial of liquid containing Bacteriostatic 0.9% Sodium Chloride Injection, USP (5 mL). After TEV-TROPIN 5mg vial is mixed with the diluent, the vial should be used within 14 days.
TEV-TROPIN 10 mg is supplied with a syringe of liquid containing vial Bacteriotstatic Water for Injection with 0.33% Metacresol as a preservative. After TEV-TROPIN 10 mg vial is mixed with the diluent, the vial should be used within 28 days.
Before preparing your medication, be sure to wash your hands thoroughly with soap and water. This will help prevent infection.
Preparing TEV-TROPIN 5 mg:
(1) Remove the hard plastic cap from the top of the diluent vial by gently pushing up on the edge of the cap. Do not remove the rubber stopper. Using an alcohol swab, wipe off the top of the diluent bottle. After cleansing, do not touch the rubber stopper.
(2) Carefully remove the needle cap from the syringe, taking care not to touch the needle. Do not discard the needle cap. Hold the barrel of the syringe with one hand and pull back on the plunger with the other, until you have drawn up the amount of air that is the same as the amount of diluent your doctor has ordered.
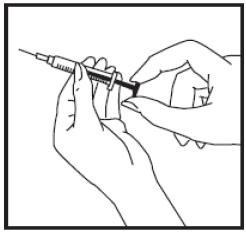
(3) Insert the needle into the diluent vial through the center of the cleansed rubber stopper. Push down on the plunger until all the air is released into the vial.
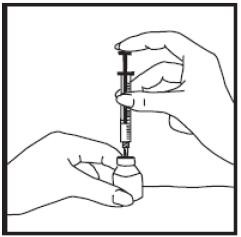
(4) Holding the vial with one hand, carefully turn it upside down making sure the syringe needle stays in the vial. The tip of the needle should be below the surface of the diluent. With your other hand, gently pull back the plunger until the amount of diluent your doctor has prescribed is in the syringe. Once the syringe is correctly filled with the diluent, remove the syringe and needle.
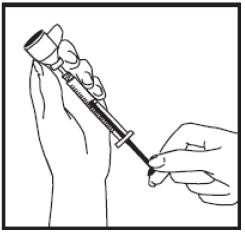
Preparing TEV-TROPIN 10mg:
Remove the syringe tip cap from the top of the pre-filled diluent syringe and attach the 25G mixing needle that is supplied in the carton.
DILUTING TEV-TROPIN
Remove the hard plastic top of the GROWTH HORMONE vial. Cleanse the top of the GROWTH HORMONE vial with an alcohol swab. Remove the needle cap from the syringe filled with diluent and insert the needle into the center of the rubber stopper on the GROWTH HORMONE vial from the vial and recap the needle.
(1) Point the needle toward the side of the vial and slowly push the plunger so that the diluent squirts onto the side of the vial, not directly onto the powder. When all the diluent is in the GROWTH HORMONE vial, remove the needle from the vial, recap the needle and discard the syringe.

(2) To mix the GROWTH HORMONE, hold the vial between your hands and gently roll it until the mixture is clear. NEVER SHAKE THE VIAL! Sometimes the vial may need to sit a few seconds before the mixture becomes clear. Do not use the mixture in the vial if it remains cloudy or you see particles floating in the mixture. If air bubbles appear, let the GROWTH HORMONE sit for a while until they disappear.
Write the date you reconstitute the GROWTH HORMONE on the vial label. The 5 mg vial must be used within 14 days whereas the 10 mg vial must be used within 28 days. Store reconstituted GROWTH HORMONE along with all unopened vials of the GROWTH HORMONE in a safe, clean place in the refrigerator. Do not freeze.
INJECTION
To prepare to give an injection of GROWTH HORMONE, begin by thoroughly washing your hands with soap and water. Check that the vial of GROWTH HORMONE you are using is clear and that the date of reconstitution is within 14 days (5 mg) or 28 days (10mg).
(1) Cleanse the top of the GROWTH HORMONE vial with an alcohol swab. After cleansing, do not touch the rubber stopper. Remove the needle cap from the syringe and insert the needle into the center of the rubber stopper on the GROWTH HORMONE vial.Gently pull back the plunger until the amount of GROWTH HORMONE solution your doctor has prescribed is in the syringe.
Once the needle is correctly filled with the solution, remove the needle from the vial. Recap the needle. If you are not experienced with filling a syringe, ask your doctor for a demonstration.
(2) Choose a site for your injection. Clean the injection site with an alcohol swab, using a circular pattern, and work from the injection site outward about 2 inches. Allow the alcohol to dry before giving the injection.
(3) Check that the correct dose is in the syringe and remove the needle cap. Hold the syringe the way you hold a pencil.
(4) With your free hand, pinch the cleansed skin between your fingers and quickly insert the needle into the skin at a 45° - 90° angle with a quick, firm wrist motion.
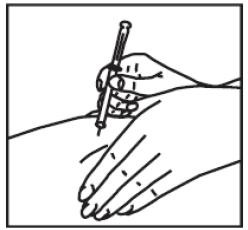
(5) Holding the syringe in place, pull back a little on the plunger and check to see if any blood flows into the syringe (vessel check). If you see blood in the syringe, remove the needle and syringe and discard. You will then need to prepare a new syringe for injection.
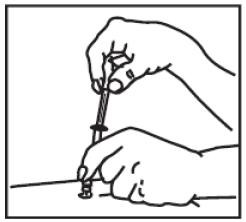
(6) If, during the vessel check, you see no blood, then slowly push down plunger until the syringe is completely empty.
(7) Quickly remove the needle from the skin and apply pressure to the injection site with a dry sterile gauze pad or cotton ball. A drop of blood may appear. Apply a small bandage if desired.
(8) Dispose of needle and syringe in a proper container identified by your physician or pharmacist. Never dispose of needles and syringes in your household trash.
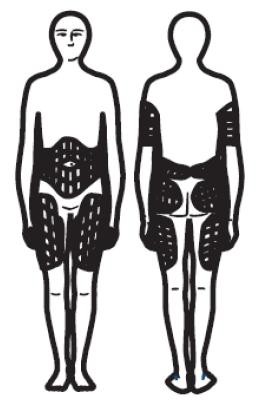
INJECTION SITES
There are different sites you can use for your injections.
These sites should be rotated.
If you notice any of the following signs, contact your physician:
-
A lump, bruise or redness at the injection site that doesn’t go away.
-
Any sign of infection at the injection site (pus, redness, heat or persistent pain).
-
Severe, sharp pain or persistent ache at injection site.
-
Rash at injection site.
Distributed By:
Teva Select Brands, Horsham, PA 19044
Division of Teva Pharmaceuticals USA
Horsham, PA 19044
Rev. B 3/2012
0082-5009v2
PACKAGE LABEL - TEV-TROPIN 5mg LABEL

NDC 57844-713-46
TEV-TROPIN™
[Somatropin (rDNA origin) for Injection]
5 mg (15 IU)
Reconstitute with Bacteriostatic 0.9% Sodium Chloride Injection, USP (Benzyl Alcohol Preserved)
GATE Rx only
PROTECT FROM LIGHT.
For Subcutaneous Use Only.
Each vial contains: 5 mg (15 IU) of somatropin lyophilized with 30 mg of mannitol.
Swirl gently to dissolve. DO NOT SHAKE.
Refrigerate at 2° to 8°C (36° to 46°F). Reconsituted vials
should be refrigerated and used within 14 days. Do Not Freeze.
Manufactured In Israel:
Distributed By:
GATE PHARMACEUTICALS
div. TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Rev. A. 9/2004 0082-1016v2
Lot No.:
Exp. Date:
PACKAGE LABEL - DILUENT 5mL LABEL

NDC 57844-103-33
Bacteriostatic 0.9% Sodium Chloride Injection, USP (Benzyl Alcohol Preserved)
NOT FOR USE IN NEWBORNS
NOT FOR INHALATION
GATE Rx only
5 mL
For Subcutaneous Use Only.
Each vial contains: 0.9% sodium chloride, 09.% benzyl alcohol, and Water for Injection, USP.
Refrigerate at 2° to 8°C (36° to 46°F).
Manufactured In Israel
Distributed By:
GATE PHARMACEUTICALS
div. TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Rev. A 9/2004 0082-6008v2
Lot No.:
Exp. Date:
PACKAGE LABEL - TEV-TROPIN 5mg CARTON

NDC 57844-713-19
TEV-TROPIN™
[Somatropin (rDNA origin) for Injection]
5 mg (15 IU)
Reconstitute with Bacteriostatic 0.9% Sodium Chloride Injection, USP
(Benzyl Alcohol Preserved)
CONTENTS:
1 vial TEV-TROPIN™ [Somatropin (rDNA origin) for Injection]
1 vial Bacteriostatic 0.9% Sodium Chloride Injection, USP
(Benzyl Alcohol Preserved)
Each vial of TEV-TROPIN™ contains 5 mg (15 IU) somatropin lyophilized with 30 mg of mannitol.
Each vial of Bacteriostatic 0.9% Sodium Chloride Injection, USP contains 0.9% Sodium Chloride, USP,
0.9% Benzyl Alcohol, USP, and Water for Inejction, USP.
Usual Dosage: See package insert.
Manufactured in Israel
Distributed By:
GATE PHARMACEUTICALS
div. of TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
GATE Rx only
PACKAGE LABEL - TEV-TROPIN 10mg LABEL
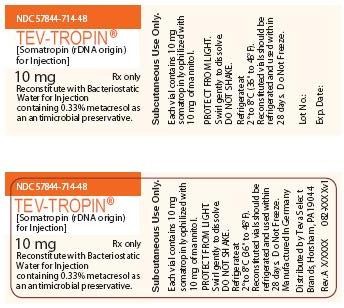
NDC 57844-714-48
TEV-TROPIN®
[Somatropin (rDNA origin) for Injection]
10 mg Rx only
Reconstitute with Bacteriostatic Water for Injection containing 0.33% metacresol as an antimicrobial preservative.
Subcutaneous Use Only.
Each vial contains 10 mg somatropin lyophilized with 10 mg of mannitol.
PROTECT FROM LIGHT
Swirl gently to dissolve.
DO NOT SHAKE.
Refrigerate at 2° to 8°C (36° to 46°F).
Reconstituted vials should be refrigerated and used within 28 days. Do Not Freeze.
Lot No.:
Exp. Date:
NDC 57844-714-48
TEV-TROPIN®
[Somatropin (rDNA origin) for Injection]
10 mg Rx only
Reconstitute with Bacteriostatic Water for Injection containing 0.33% metacresol as an antimicrobial preservative.
Subcutaneous Use Only.
Each vial contains 10 mg somatropin lyophilized with 10 mg of mannitol.
PROTECT FROM LIGHT
Swirl gently to dissolve.
DO NOT SHAKE.
Refrigerate at 2° to 8°C (36° to 46°F).
Reconstituted vials should be refrigerated and used within 28 days. Do Not Freeze.
Manufactured in Germany
TEVA Biologics & Specialty Products
Horsham, PA 19044
Rev. A X/XXXX 082-XXXXv1
PACKAGE LABEL - DILUENT 1mL LABEL
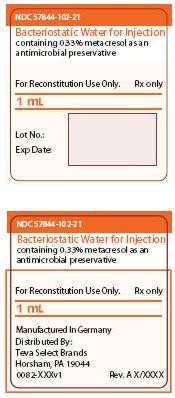
NDC 57844-102-21
Bacteriostatic Water for Injection
containing 0.33% metacresol as an antimicrobial preservative
For Reconstitution Use Only. Rx only
1 mL
Lot No.:
Exp Date:
NDC 57844-102-21
Bacteriostatic Water for Injection
containing 0.33% metacresol as an antimicrobial preservative
For Reconstitution Use Only. Rx only
1 mL
Manufactured In Germany
Distributed By:
TEVA Biologics & Specialty Products
Horsham, PA 19044
0082-XXXv1 Rev. A X/XXXX
PACKAGE LABEL - 10 mg CARTON

NDC 57844-714-19
TEV-TROPIN®
[somatropin (rDNA origin) for injection]
10 mg
CONTENTS:
1 vial TEV-TROPIN® [somatropin (rDNA origin) for Injection]
1 prefilled diluent syringe
1 needle attachment
Each vial of TEV-TROPIN™ contains 10 mg somatropin lyophilized with 10 mg of mannitol.
Each prefilled syringe contains 1 mL of Bacteriostatic Water for Injection, containing
0.33% metacresol as an antimicrobial preservative. The reconstituted solution has a pH of 7.0 - 9.0
Usual Dosage: See package insert.
Manufactured in Germany
TEVA
TEVA BIOLOGICS & SPECIALTY PRODUCTS
Marketed By:
Teva Select Brands
Horsham, PA 19044
Rx only
TEV-TROPINsomatropin KIT
| |||||||||||||||||||||||||||||||||||||||||||||
TEV-TROPINsomatropin KIT
| |||||||||||||||||||||||||||||||||||||||||||||