Thalamus compositum
Thalamus compositum oral vial
FULL PRESCRIBING INFORMATION: CONTENTS*
- THALAMUS COMPOSITUM DESCRIPTION
- INDICATION AND USAGE
- THALAMUS COMPOSITUM DOSAGE AND ADMINISTRATION
- THALAMUS COMPOSITUM CONTRAINDICATIONS
- WARNINGS AND PRECAUTIONS
- THALAMUS COMPOSITUM ADVERSE REACTIONS
- OVERDOSAGE
- CLINICAL PHARMACOLOGY
- DOSAGE
FULL PRESCRIBING INFORMATION
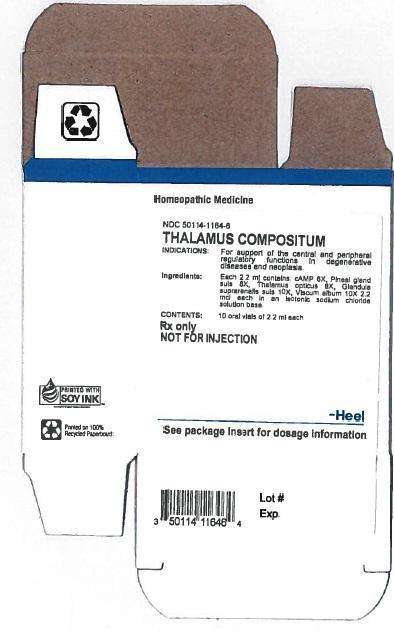
THALAMUS COMPOSITUM DESCRIPTION
Each 2.2 ml ampule contains:
|
Ingredient Name |
Potency |
Quantity |
|
cAMP |
6x |
22 |
|
Pineal gland suis |
8x |
22µ |
|
Thalamus opticus |
8x |
22µ |
|
Glandula suprarenalis suis |
10x |
22µ |
|
Viscum album |
10x |
22µ |
Inactive Ingredient: Isotonic Sodium Chloride solution
INDICATION AND USAGE
Thalamus compositum® Oral Vials is a homeopathic drug product indicated for the support of the central and peripheral regulatory functions in degenerative diseases and neoplasia
THALAMUS COMPOSITUM DOSAGE AND ADMINISTRATION
Dosage:
Adults and children above 6 years: 1 vial orally 1-3 times daily
Children up to 6 years: ½ vial orally 1-3 times daily
THALAMUS COMPOSITUM CONTRAINDICATIONS
Thalamus compositum® Oral Vials are contraindicated in patients with known hypersensitivity to Thalamus compositum®or any of its ingredients.
WARNINGS AND PRECAUTIONS
Warnings and Precautions
None
THALAMUS COMPOSITUM ADVERSE REACTIONS
No adverse events have been reported with a causal relationship to Thalamus compositum® Oral Vials.
OVERDOSAGE
Overdosage: No negative effects of an overdose have been reported and none are expected due to the homeopathic dilutions
CLINICAL PHARMACOLOGY
Mechanism of Action
The exact mechanism of Thalamus compositum®Oral Vials is not fully understood.
Pharmacodynamics
Not applicable for homeopathic medicinal products.
DOSAGE
1 oral vial containing 2.2ml solution for oral administration
Thalamus compositumADENOSINE CYCLIC PHOSPHATE, SUS SCROFA PINEAL GLAND, SUS SCROFA THALAMUS LATERAL GENICULATE NUCLEUS, SUS SCROFA ADRENAL GLAND and VISCUM ALBUM FRUITING TOP SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||