Thallous Chloride Tl 201
GE Healthcare, Medi-Physics, Inc.
THALLOUS CHLORIDETL 201 INJECTION
FULL PRESCRIBING INFORMATION: CONTENTS*
- THALLOUS CHLORIDE TL 201 DESCRIPTION
- CLINICAL PHARMACOLOGY
- THALLOUS CHLORIDE TL 201 INDICATIONS AND USAGE
- THALLOUS CHLORIDE TL 201 CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- THALLOUS CHLORIDE TL 201 ADVERSE REACTIONS
- THALLOUS CHLORIDE TL 201 DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
Rx ONLY
Product Number: 2097, 2098
DIAGNOSTIC – FOR INTRAVENOUS USE
THALLOUS CHLORIDE TL 201 DESCRIPTION
Thallous Chloride Tl 201 Injection is supplied in isotonic solution as a sterile, nonpyrogenic, diagnostic radiopharmaceutical for intravenous administration. Each unit dose contains 1 mL and each milliliter contains 37 MBq (1 mCi) of Thallous Chloride Tl 201 Injection at calibration time. The pH is adjusted to 4.5-7.5 with hydrochloric acid or sodium hydroxide. It is made isotonic with 9 mg sodium chloride/mL and is preserved with 0.009 mL benzyl alcohol/mL.
Thallium Tl 201 is cyclotron produced with no carrier added. The radionuclidic composition at calibration time, expressed as percent of total activity, is not less than 98 percent Thallium Tl 201 with not more than 0.3 percent Thallium Tl 200 , not more than 1.2 percent Thallium Tl 202, not more than 0.2 percent Lead Pb 203, and not more than 0.3 percent all others.
The concentration of each radionuclidic contaminant changes with time. Therefore, it is recommended that Thallous Chloride Tl 201 Injection be administered close to calibration time to minimize the effect of higher levels of radionuclidic contaminants pre and post calibration. Graph 1 shows maximum allowable concentration of each radionuclidic contaminant as a function of time.
Graph 1. Radionuclidic Contaminants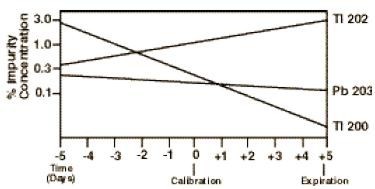
PHYSICAL CHARACTERISTICS
Thallium Tl 201 decays by electron capture to Mercury Hg 201 with a physical half-life of 73.1 hours. Photons that are useful for detection and imaging are listed in Table 1. The lower energy x-rays obtained from the Mercury Hg 201 daughter of Tl 201 are recommended for myocardial imaging because the mean %/disintegration at 68.9-80.3 keV is much greater than the combination of gamma-4 and gamma-6 mean %/disintegration.
| Radiation | Mean %/Disintegration | Mean Energy (keV) |
|---|---|---|
| Gamma-4 | 2.7 | 135.3 |
| Gamma-6 | 10.0 | 167.4 |
| Mercury x-rays | 94.4 | 68.9-80.3 |
EXTERNAL RADIATION
The specific gamma ray constant for Thallium Tl 201 is 3.21 microcoulombs/hr-kg-MBq (0.46 R/hr-mCi) at 1 cm. The first half-value layer is 0.023 cm of lead. A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from the interposition of various thicknesses of lead (Pb) is shown in Table 2. For example, the use of 0.31 cm of lead will decrease the external radiation exposure by a factor of about 1,000.
| cm of Lead (Pb) | Coefficient of Attenuation |
|---|---|
| 0.023 | 0.5 |
| 0.081 | 10-1 |
| 0.19 | 10-2 |
| 0.31 | 10-3 |
| 0.44 | 10-4 |
To correct for physical decay of this radionuclide, the fractions that remain at selected intervals and after calibration are shown in Table 3.
| Hours | Fraction Remaining | Hours | Fraction Remaining | Hours | Fraction Remaining |
|---|---|---|---|---|---|
| 0 | 1.00 | 42 | 0.67 | 84 | 0.45 |
| 6 | 0.94 | 48 | 0.63 | 90 | 0.43 |
| 12 | 0.89 | 54 | 0.60 | 96 | 0.40 |
| 18 | 0.84 | 60 | 0.57 | 108 | 0.36 |
| 24 | 0.80 | 66 | 0.53 | 120 | 0.32 |
| 30 | 0.75 | 72 | 0.51 | ||
| 36 | 0.71 | 78 | 0.48 |
At two and four days post calibration, Thallium Tl 201 concentrations amount only to about 63% and 40%, respectively, of their initial value. This condition would require use of proportionately larger volume doses.
CLINICAL PHARMACOLOGY
Thallous Chloride Tl 201 Injection with no carrier added has been found to accumulate in viable myocardium in a manner analogous to that of potassium. Experiments employing labeled microspheres in human volunteers have shown that the myocardial distribution of Thallous Chloride Tl 201 Injection correlates well with regional perfusion.
In clinical studies, thallium images have been found to visualize areas of infarction as "cold" or nonlabeled regions which are confirmed by electrocardiographic and enzyme changes. Regions of transient myocardial ischemia corresponding to areas perfused by coronary arteries with partial stenoses have been visualized when thallium was administered in conjunction with an exercise stress test.
Intravenous administration of Thallous Chloride Tl 201 Injection is characterized by rapid biexponential clearance from the blood with about 91.5% of blood radioactivity disappearing with a half-life of approximately 5 minutes and the remainder with a half-life of about 40 hours. Maximal concentration by normal myocardium occurs at about ten minutes with sustained myocardial retention and adequate concentration in heart muscle to permit gated imaging. In addition, localization occurs in parathyroid adenomas and to a lesser extent in sites of parathyroid hyperplasia and other abnormal tissues such as thyroid adenoma, neoplasia (e.g. parathyroid carcinoma) and sarcoid. Biodistribution is generally proportional to organ blood flow at the time of injection. Blood clearance of Thallous Chloride Tl 201 Injection is primarily by the myocardium, kidneys, thyroid, liver and stomach with the remainder distributing fairly uniformly throughout the body. The dosimetry data in Table 4 reflect this distribution pattern and are based on a biological half-life of 11 days and an effective half-life of 2.4 days. Thallous Chloride Tl 201 Injection is excreted slowly and to an equal extent in both feces and urine.
THALLOUS CHLORIDE TL 201 INDICATIONS AND USAGE
Thallous Chloride Tl 201 may be useful in myocardial perfusion imaging for the diagnosis and localization of myocardial infarction. It may also have prognostic value regarding survival, when used in the clinically stable patient following the onset of symptoms of an acute myocardial infarction, to assess the site and size of the perfusion defect.
Thallous Chloride Tl 201 may also be useful in conjunction with exercise stress testing as an adjunct in the diagnosis of ischemic heart disease (atherosclerotic coronary artery disease) .
It is usually not possible to differentiate recent from old myocardial infarction, or to differentiate exactly between recent myocardial infarction and ischemia.
Thallous Chloride Tl 201 is indicated also for the localization of sites of parathyroid hyperactivity in patients with elevated serum calcium and parathyroid hormone levels. It may also be useful in pre-operative screening to localize extrathyroidal and mediastinal sites of parathyroid hyperactivity and for post-surgical reexamination. Thallous Chloride Tl 201 has not been adequately demonstrated to be effective for the localization of normal parathyroid glands.
THALLOUS CHLORIDE TL 201 CONTRAINDICATIONS
None known.
WARNINGS
When studying patients suspected or known to have myocardial infarction or ischemia, care should be taken to assure continuous clinical monitoring and treatment in accordance with safe, accepted procedure. Exercise stress testing should be performed only under the supervision of a qualified physician and in a laboratory equipped with appropriate resuscitation and support apparatus.
The contents of this vial are radioactive. Adequate shielding of the preparation must be maintained at all times.
PRECAUTIONS
Data are not available concerning the effect on the quality of Thallous Chloride Tl 201 Injection scans of marked alterations in blood glucose, insulin, or pH (such as is found in diabetes mellitus). Attention is directed to the fact that thallium is a potassium analog, and since the transport of potassium is affected by these factors, the possibility exists that the thallium may likewise be affected.
General
Do not use after the expiration time and date (5 days maximum after calibration time) stated on the label.
Do not use if contents are turbid.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
Thallous Chloride Tl 201 Injection, as well as other radioactive drugs, must be handled with care and appropriate safety measures should be used to minimize radiation exposure to clinical personnel. Also, care should be taken to minimize radiation exposure to patients in a manner consistent with proper patient management.
This radiopharmaceutical is licensed by the Illinois Emergency Management Agency for distribution to persons licensed pursuant to 32 Ill. Admin. Code Section 330.260(a) and Section 335, Subpart D, 335.3010 and Subpart E, 335.4010 or under equivalent licenses of an Agreement State or a Licensing State.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential, mutagenic potential, or whether Thallous Chloride Tl 201 Injection affects fertility in males or females. Ideally, examinations using radiopharmaceuticals, especially those elective in nature, of a woman of childbearing capability should be performed during the first ten days following the onset of menses.
Pregnancy Category C
Animal reproduction studies have not been conducted with Thallous Chloride Tl 201 Injection. It is also not known whether Thallous Chloride Tl 201 Injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Thallous Chloride Tl 201 Injection should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It has been found that this drug is excreted in human milk during lactation. Therefore, formula feedings should be substituted for breast feedings.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 18 have not been established.
Geriatric Use
Clinical studies of Thallous Chloride TI 201 did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
THALLOUS CHLORIDE TL 201 ADVERSE REACTIONS
Adverse reactions that have been reported with the administration of Thallous Chloride Tl 201 Injection include allergic-type skin reactions, pruritus, itching, hypotension, nausea, sweating, and blurred vision.
THALLOUS CHLORIDE TL 201 DOSAGE AND ADMINISTRATION
The recommended adult (70 kg) dose of Thallous Chloride Tl 201 Injection is 37-74 MBq (1-2 mCi). Thallous Chloride Tl 201 Injection is intended for intravenous administration only.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
Aseptic procedures should be employed in the withdrawal of the dose for patient administration.
For resting thallium studies, imaging should begin 10-20 minutes after injection. Myocardial-to-background ratios are improved when patients are injected upright and in the fasting state; the upright position reduces the hepatic and gastric Thallium Tl 201 concentration.
When utilized in conjunction with exercise stress testing, Thallous Chloride Tl 201 Injection should be administered at the inception of a period of maximum stress which is sustained for approximately 30 seconds after injection. Imaging should begin within 10 minutes after administration to obtain maximum target-to-background ratios. Several investigators have reported that within two hours after the completion of stress testing the target-to-background ratios may decrease significantly in lesions that are attributable to transient ischemia.
For the localization of parathyroid hyperactivity, Thallous Chloride Tl 201 may be administered before, with or after a minimal dose of a thyroid imaging agent such as sodium pertechnetate Tc99m or sodium iodide I 123 to enable thyroid subtraction imaging.
RADIATION DOSIMETRY
The estimated absorbed doses at calibration time to an average patient (70 kg) from an intravenous injection of a maximum dose of 74 MBq, 2 mCi of Thallous Chloride Tl 201 Injection are shown in Table 4.
| Tissue | mGy/74 MBq | Rads/2 mCi |
|---|---|---|
| Heart Wall | 10.0 | 1.0 |
| Liver | 12.0 | 1.2 |
| Kidneys | 24.0 | 2.4 |
| Testes | 11.0 | 1.1 |
| Ovaries | 9.6 | 0.96 |
| Thyroid | 9.6 | 0.96 |
| Gastrointestinal Tract: | ||
| Stomach Wall | 8.2 | 0.82 |
| Small Intestine | 7.8 | 0.78 |
| Upper Large Intestine Wall | 5.2 | 0.52 |
| Lower Large Intestine Wall | 4.4 | 0.44 |
| Total Body | 4.4 | 0.44 |
HOW SUPPLIED
Thallous Chloride Tl 201 Injection for intravenous administration is supplied as a sterile, nonpyrogenic solution containing at calibration time, 37 MBq/mL (1 mCi/mL) of Thallous Chloride Tl 201 Injection, 9 mg sodium chloride/mL and 0.009 mL of benzyl alcohol/mL. The pH is adjusted with hydrochloric acid and/or sodium hydroxide solution. Vials are available in the following quantities of radioactivity: 244.2 and 325.6 MBq, 6.6 and 8.8 mCi of Thallous Chloride Tl 201 Injection.
NDC 17156-299-16 (6.6 mCi)
NDC 17156-299-18 (8.8 mCi)
Store in a lead shield at room temperature 15°-30°C (59°-86°F).
Preparation and Handling Procedures for Thallous Chloride Tl 201 Injection
- Waterproof gloves should be worn during the handling and injection period.
- Adequate shielding during the life of the radioactive drug should be maintained by using the lead shield and cover and by using a syringe shield for withdrawing and injecting Thallous Chloride Tl 201 Injection.
GE Healthcare
Medi-Physics, Inc.,
3350 North Ridge Avenue
Arlington Heights, IL 60004
Distributed in Canada by
GE Healthcare Canada Inc.
2300 Meadowvale Blvd
Mississauga, ON L5N 5P9
GE and the GE Monogram are trademarks of General Electric Company.
43-2090H
Revised April 2006
Thallous Chloride Tl 201Thallous Chloride, Tl-201 INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||