TheraPlus
Zhejiang Jingwei Pharmaceutical Co., Ltd.
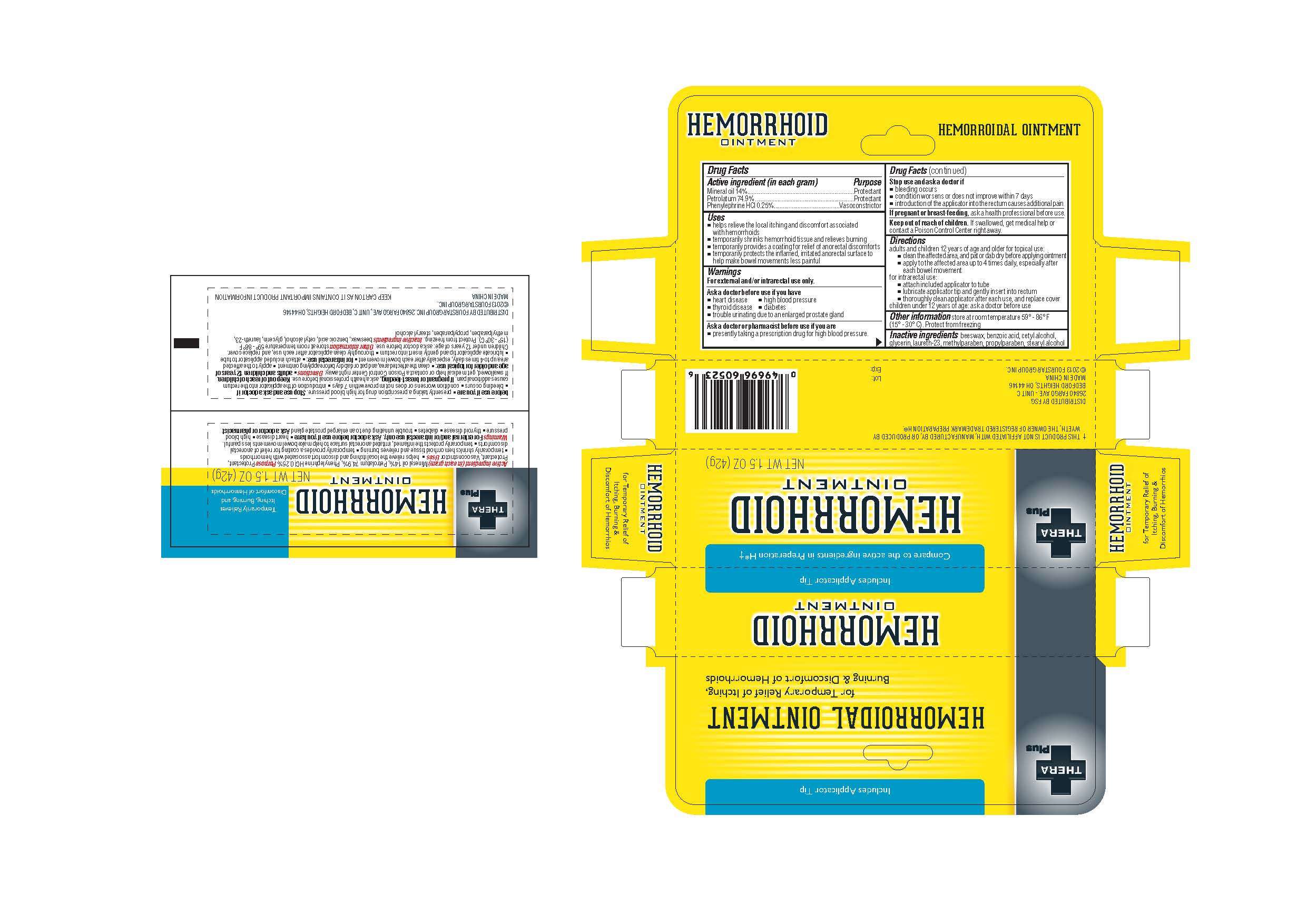
Drug Facts
FULL PRESCRIBING INFORMATION
Inactive Ingredients beeswax, benzoic acid, cetyl alcohol, glycerin, laureth-23, methylparaben, propylparaben, stearyl alcohol
Active ingredient
Active Ingredient (in each gram)
Mineral oil 14%
Petrolatum 74.9%
Phenylephrine HCI 0.25%
Purpose
Purpose
Protectant
Protectant
Vasoconstrictor
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Uses
Uses
-helps relieve the local itching and discomfort associated with hemorrhoids
-temporarily shrinks hemorrhoid tissue and relieves burning
-temporarily provides a coating for relief of anorectal discomforts
-temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful
Directions
adults and children 12 years of age and older for topical use:
-clean the affected area, and oat or dab dry before applying ointment
-apply to the affected area up to 4 times daily, especially after each bowel movement
for intrarectal use:
-attach included applicator to tube
-lubricate applicator tip and gently insert into rectum
-thoroughly clean applicator after each use, and replace cover
children under 12 years of age: ask a doctor before use
If pregnant or breast-feeding, ask a health professional before use
Stop use and ask a doctor if
-bleeding occurs
-condition worsens or does not improve within 7 days
-introduction of the applicator into the rectum causes additional pain
Ask a doctor before use if you have
-heart disease
-high blood pressure
-thyroid disease
-diabetes
-trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
-presently taking a prescription drug for high blood pressure
TheraPlusPhenylephrine HCI OINTMENT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||