Theratramadol-90
Theratramadol-90
FULL PRESCRIBING INFORMATION
DESCRIPTION Tramadol hydrochloride tablet is a centrally acting analgesic. The chemical name for tramadol hydrochloride is (±) cis-2-[(dimethylamino)methyl]-1-(3-methoxyphenyl) cyclohexanol hydrochloride. Its structural formula is:
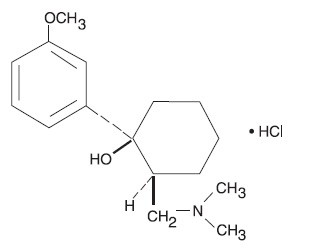
Molecular formula is C16H13NO2•HCl The molecular weight of tramadol hydrochloride is 299.8. Tramadol hydrochloride is a white, bitter, crystalline and odorless powder. It is readily soluble in water and ethanol and has a pKa of 9.41. The n-octanol/water log partition coefficient (logP) is 1.35 at pH7. Tramadol hydrochloride tablets, for oral administration contain 50 mg of tramadol hydrochloride. In addition, each tablet contains the following inactive ingredients: pregelatinized starch, lactose anhydrous, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sodium starch glycolate, titanium dioxide.
CLINICAL PHARMACOLOGY Pharmacodynamics
Tramadol hydrochloride is a centrally acting synthetic opioid analgesic. Although its mode of action is not completely understood, from animal tests, at least two complementary mechanisms appear applicable: binding of parent and M1 metabolite to µ-opioid receptors and weak inhibition of reuptake of norepinephrine and serotonin. Opioid activity is due to both low affinity binding of the parent compound and higher affinity binding of the O-demethylated metabolite M1 to µ-opioid receptors. In animal models, M1 is up to 6 times more potent than tramadol in producing analgesia and 200 times more potent in µ-opioid binding. Tramadol-induced analgesia is only partially antagonized by the opiate antagonist naloxone in several animal tests. The relative contribution of both tramadol and M1 to human analgesia is dependent upon the plasma concentrations of each compound (see CLINICAL PHARMACOLOGY, Pharmacokinetics). Tramadol has been shown to inhibit reuptake of norepinephrine and serotonin in vitro, as have some other opioid analgesics. These mechanisms may contribute independently to the overall analgesic profile of tramadol hydrochloride tablets. Analgesia in humans begins approximately within one hour after administration and reaches a peak in approximately two to three hours. Apart from analgesia, tramadol hydrochloride tablets administration may produce a constellation of symptoms (including dizziness, somnolence, nausea, constipation, sweating and pruritus) similar to that of other opioids. In contrast to morphine, tramadol has not been shown to cause histamine release. At therapeutic doses, tramadol hydrochloride tablets have no effect on heart rate, left-ventricular function or cardiac index. Orthostatic hypotension has been observed.
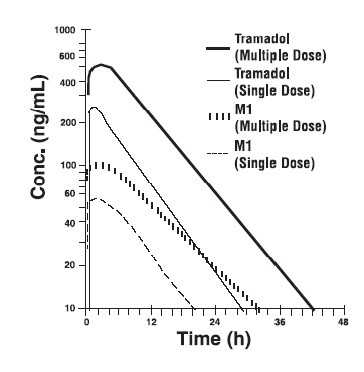
| Population/ Dosage Regimena | Parent Drug/ Metabolite | Peak Conc. (ng/mL) | Time to Peak (hrs) | Clearance/Fb (mL/min/kg) | t 1/2 (hrs) |
| Healthy Adults, 100 mg q.i.d., MD p.o. | Tramadol | 592 (30) | 2.3 (61) | 5.90 (25) | 6.7 (15) |
| M1 | 110 (29) | 2.4 (46) | c | 7.0 (14) | |
| Healthy Adults, 100 mg SD p.o. | Tramadol | 308 (25) | 1.6 (63) | 8.50 (31) | 5.6 (20) |
| M1 | 55.0 (36) | 3.0 (51) | c | 6.7 (16) | |
| Geriatric, (greater than 75 yrs) 50 mg SD p.o. | Tramadol | 208 (31) | 2.1 (19) | 6.89 (25) | 7.0 (23) |
| M1 | d | d | c | ||
| Hepatic Impaired, 50 mg SD p.o. | Tramadol | 217 (11) | 1.9 (16) | 4.23 (56) | d13.3 (11) |
| M1 | 19.4 (12) | 9.8 (20) | c | 18.5 (15) | |
| Renal Impaired, CLcr10-30 mL/min 100 mg SD i.v | Tramadol | c | c | 4.23 (54) | 10.6 (31) |
| M1 | c | c | c | 11.5 (40) | |
| Renal Impaired, CLcr less than 5mL/min 100 mg SD i.v | Tramadol | c | c | 3.73 (17) | 11.0 (29) |
| M1 | c | c | c | 16.9 (18) |
Uses
INDICATIONS AND USAGE Tramadol hydrochloride tablets are indicated for the management of moderate to moderately severe pain in adults.
CONTRAINDICATIONS Tramadol hydrochloride tablets should not be administered to patients who have previously demonstrated hypersensitivity to tramadol, any other component of this product or opioids. Tramadol hydrochloride is contraindicated in any situation where opioids are contraindicated, including acute intoxication with any of the following: alcohol, hypnotics, narcotics, centrally acting analgesics, opioids or psychotropic drugs. Tramadol may worsen central nervous system and respiratory depression in these patients.
Seizure Risk Seizures have been reported in patients receiving tramadol hydrochloride tablets within the recommended dosage range. Spontaneous post-marketing reports indicate that seizure risk is increased with doses of tramadol hydrochloride tablets above the recommended range. Concomitant use of tramadol hydrochloride tablets increases the seizure risk in patients taking: • Selective serotonin reuptake inhibitors (SSRI antidepressants or anoretics), • Tricyclic antidepressants (TCAs), and other tricyclic compounds (e.g., cyclobenzaprine, promethazine, etc.), or • Other opioids. Administration of tramadol hydrochloride tablets may enhance the seizure risk in patients taking: • MAO inhibitors (see also WARNINGS - Use with MAO inhibitors), • Neuroleptics, or • Other drugs that reduce the seizure threshold. Risk of convulsions may also increase in patients with epilepsy, those with a history of seizures, or in patients with a recognized risk for seizure (such as head trauma, metabolic disorders, alcohol and drug withdrawal, CNS infections). In tramadol hydrochloride tablets overdose, naloxone administration may increase the risk of seizure. Anaphylactoid Reactions Serious and rarely fatal anaphylactoid reactions have been reported in patients receiving therapy with tramadol hydrochloride tablets. When these events do occur it is often following the first dose. Other reported allergic reactions include pruritus, hives, bronchospasm, and angioedema, toxic epidermal necrolysis and Stevens Johnson syndrome. Patients with a history of anaphylactoid reactions to codeine and other opioids may be at increased risk and therefore should not receive tramadol hydrochloride tablets (see CONTRAINDICATIONS). Respiratory Depression Administer tramadol hydrochloride tablets cautiously in patients at risk for respiratory depression. In these patients alternative non-opioid analgesics should be considered. When large doses of tramadol hydrochloride tablets are administered with anesthetic medications or alcohol, respiratory depression may result. Respiratory depression should be treated as an overdose. If naloxone is to be administered, use cautiously because it may precipitate seizures (see WARNINGS, Seizure Risk and OVERDOSAGE). Interaction with Central Nervous System (CNS) Depressants Tramadol should be used with caution and in reduced dosages when administered to patients receiving CNS depressants such as alcohol, opioids, anesthetic agents, narcotics, phenothiazines, tranquilizers or sedative hypnotics. Tramadol increased the risk of CNS and respiratory depression in these patients. Increased Intracranial Pressure or Head Trauma Tramadol hydrochloride tablets should be used with caution in patients with increased intracranial pressure or head injury. The respiratory depressant effects of opioids include carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure, and may be markedly exaggerated in those patients. Additionally, pupillary changes (miosis) from tramadol may obscure the existence, extent, or course of intracranial pathology. Clinicians should also maintain a high index of suspicion for adverse drug reaction when evaluating altered mental status in these patients if they are receiving tramadol hydrochloride tablets. (See Respiratory Depression) Use in Ambulatory Patients Tramadol may impair the mental and or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. The patients using this drug should be cautioned accordingly.Enter section text here
Use with MAO Inhibitors and serotonin re-uptake inhibitors Use tramadol hydrochloride tablets with great caution in patients taking monoamine oxidase inhibitors. Animal studies have shown increased deaths with combined administration. Concomitant use of tramadol hydrochloride tablets with MAO inhibitors or SSRI's increases the risk of adverse events, including seizure and serotonin syndrome. Withdrawal Withdrawal symptoms may occur if tramadol hydrochloride tablets are discontinued abruptly. (See DRUG ABUSE and DEPENDENCE) These symptoms may include: anxiety, sweating, insomnia, rigors, pain, nausea, tremors, diarrhea, upper respiratory symptoms, piloerection, and rarely hallucinations. Clinical experience suggests that withdrawal symptoms may be relieved by tapering the medication.
Physical Dependence and Abuse Tramadol hydrochloride tablets may induce psychic and physical dependence of the morphine-type (µ-opioid) (See DRUG ABUSE and DEPENDENCE). Tramadol hydrochloride tablets should not be used in opioid-dependent patients. Tramadol hydrochloride has been shown to reinitiate physical dependence in some patients that have been previously dependent on other opioids. Dependence and abuse, including drug-seeking behavior and taking illicit actions to obtain the drug, are not limited to those patients with prior history of opioid dependence.
Risk of Overdosage Serious potential consequences of overdosage with tramadol hydrochloride tablets are central nervous system depression, respiratory depression and death. In treating an overdose, primary attention should be given to maintaining adequate ventilation along with general supportive treatment (See OVERDOSAGE).
PRECAUTIONS Acute Abdominal Conditions The administration of tramadol hydrochloride tablets may complicate the clinical assessment of patients with acute abdominal conditions. Use in Renal and Hepatic Disease Impaired renal function results in a decreased rate and extent of excretion of tramadol and its active metabolite, M1. In patients with creatinine clearances of less than 30 mL/min, dosing reduction is recommended (see DOSAGE AND ADMINISTRATION). Metabolism of tramadol and M1 is reduced in patients with advanced cirrhosis of the liver. In cirrhotic patients, dosing reduction is recommended (see DOSAGE AND ADMINISTRATION). With the prolonged half-life in these conditions, achievement of steady-state is delayed, so that it may take several days for elevated plasma concentrations to develop.
Information for Patients • Tramadol hydrochloride tablets may impair mental or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. • Tramadol hydrochloride tablets should not be taken with alcohol containing beverages. • Tramadol hydrochloride tablets should be used with caution when taking medications such as tranquilizers, hypnotics or other opiate containing analgesics. • The patient should be instructed to inform the physician if they are pregnant, think they might become pregnant, or are trying to become pregnant (see PRECAUTIONS: Labor and Delivery). • The patient should understand the single-dose and 24-hour dose limit and the time interval between doses, since exceeding these recommendations can result in respiratory depression seizures and death.
Drug Interactions In vitro studies indicate that tramadol is unlikely to inhibit the CYP3A4-mediated metabolism of other drugs when tramadol is administered concomitantly at therapeutic doses. Tramadol does not appear to induce its own metabolism in humans, since observed maximal plasma concentrations after multiple oral doses are higher than expected based on single-dose data. Tramadol is a mild inducer of selected drug metabolism pathways measured in animals. Use with Carbamazepine Patients taking carbamazepine may have a significantly reduced analgesic effect of tramadol hydrochloride tablets. Because carbamazepine increases tramadol metabolism and because of the seizure risk associated with tramadol, concomitant administration of tramadol hydrochloride tablets and carbamazepine is not recommended. Use with Quinidine Tramadol is metabolized to M1 by CYP2D6. Quinidine is a selective inhibitor of that isoenzyme, so that concomitant administration of quinidine and tramadol hydrochloride tablets results in increased concentrations of tramadol and reduced concentrations of M1.The clinical consequences of these findings are unknown. In vitro drug interaction studies in human liver microsomes indicate that tramadol has no affect on quinidine metabolism. Use with Inhibitors of CYP2D6 In vitro drug interaction studies in human liver microsomes indicate that concomitant administration with inhibitors of CYP2D6 such as fluoxetine, paroxetine, and amitriptyline could result in some inhibition of the metabolism of tramadol. Use with Cimetidine Concomitant administration of tramadol hydrochloride tablets with cimetidine does not result in clinically significant changes in tramadol pharmacokinetics. Therefore, no alteration of the tramadol hydrochloride tablets dosage regimen is recommended. Use with MAO Inhibitors Interactions with MAO Inhibitors, due to interference with detoxification mechanisms, have been reported for some centrally acting drugs (see WARNINGS, Use with MAO inhibitors). Use with Digoxin and Warfarin Post-marketing surveillance has revealed rare reports of digoxin toxicity and alteration of warfarin effect, including elevation of prothrombin times.
Carcinogenesis, Mutagenesis, Impairment of Fertility A slight, but statistically significant, increase in two common murine tumors, pulmonary and hepatic, was observed in a mouse carcinogenicity study, particularly in aged mice. Mice were dosed orally up to 30 mg/kg (90 mg/m2 or 0.36 times the maximum daily human dosage of 246 mg/m2) for approximately two years, although the study was not done with the Maximum Tolerated Dose. This finding is not believed to suggest risk in humans. No such finding occurred in a rat carcinogenicity study (dosing orally up to 30 mg/kg, 180 mg/m2, or 0.73 times the maximum daily human dosage). Tramadol was not mutagenic in the following assays: Ames Salmonella microsomal activation test, CHO/HPRT mammalian cell assay, mouse lymphoma assay (in the absence of metabolic activation), dominant lethal mutation tests in mice, chromosome aberration test in Chinese hamsters, and bone marrow micronucleus tests in mice and Chinese hamsters. Weakly mutagenic results occurred in the presence of metabolic activation in the mouse lymphoma assay and micronucleus test in rats. Overall, the weight of evidence from these tests indicates that tramadol does not pose a genotoxic risk to humans.
No effects on fertility were observed for tramadol at oral dose levels up to 50 mg/kg (300 mg/m2) in male rats and 75 mg/kg (450 mg/m2) in female rats. These dosages are 1.2 and 1.8 times the maximum daily human dosage of 246 mg/m2, respectively.
Pregnancy, Teratogenic Effects: Pregnancy Category C Tramadol has been shown to be embryotoxic and fetotoxic in mice, (120 mg/kg or 360 mg/m2), rats (≥ 25 mg/kg or 150 mg/m2) and rabbits (≥ 75 mg/kg or 900 mg/m2) at maternally toxic dosages but was not teratogenic at these dose levels. These dosages on a mg/m2 basis are 1.4, ≥ 0.6, and ≥ 3.6 times the maximum daily human dosage (246 mg/m2) for mouse, rat and rabbit, respectively. No drug-related teratogenic effects were observed in progeny of mice (up to 140 mg/kg or 420 mg/m2), rats (up to 80 mg/kg or 480 mg/m2) or rabbits (up to 300 mg/kg or 3600 mg/m2) treated with tramadol by various routes. Embryo and fetal toxicity consisted primarily of decreased fetal weights, skeletal ossification and increased supernumerary ribs at maternally toxic dose levels. Transient delays in developmental or behavioral parameters were also seen in pups from rat dams allowed to deliver. Embryo and fetal lethality were reported only in one rabbit study at 300 mg/kg (3600 mg/m2), a dose that would cause extreme maternal toxicity in the rabbit. The dosages listed for mouse, rat and rabbit are 1.7, 1.9 and 14.6 times the maximum daily human dosage (246 mg/m2), respectively. Non-teratogenic Effects Tramadol was evaluated in peri- and post-natal studies in rats. Progeny of dams receiving oral (gavage) dose levels of 50 mg/kg (300 mg/m2 or 1.2 times the maximum daily human tramadol dosage) or greater had decreased weights, and pup survival was decreased early in lactation at 80 mg/kg (480 mg/m2 or 1.9 and higher the maximum daily human dose). There are no adequate and well-controlled studies in pregnant women. Tramadol hydrochloride should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Neonatal seizures, neonatal withdrawal syndrome, fetal death and still birth have been reported during post-marketing.
Nursing Mothers Tramadol hydrochloride is not recommended for obstetrical preoperative medication or for post-delivery analgesia in nursing mothers because its safety in infants and newborns has not been studied. Following a single IV 100 mg dose of tramadol, the cumulative excretion in breast milk within 16 hours postdose was 100 mcg of tramadol (0.1% of the maternal dose) and 27 mcg of M1.
Pediatric Use The safety and efficacy of tramadol hydrochloride in patients under 16 years of age have not been established. The use of tramadol hydrochloride in the pediatric population is not recommended.
Geriatric Use In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy. In patients over 75 years of age, daily doses in excess of 300 mg are not recommended (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION). A total of 455 elderly (65 years of age or older) subjects were exposed to tramadol hydrochloride in controlled clinical trials. Of those, 145 subjects were 75 years of age and older.
In studies including geriatric patients, treatment-limiting adverse events were higher in subjects over 75 years of age compared to those under 65 years of age. Specifically, 30% of those over 75 years of age had gastrointestinal treatment-limiting adverse events compared to 17% of those under 65 years of age. Constipation resulted in discontinuation of treatment in 10% of those over 75.
ADVERSE REACTIONS Tramadol hydrochloride was administered to 550 patients during the double-blind or open-label extension periods in U.S. studies of chronic nonmalignant pain. Of these patients, 375 were 65 years old or older. Table 2 reports the cumulative incidence rate of adverse reactions by 7, 30 and 90 days for the most frequent reactions (5% or more by 7 days). The most frequently reported events were in the central nervous system and gastrointestinal system. Although the reactions listed in the table are felt to be probably related to tramadol hydrochloride administration, the reported rates also include some events that may have been due to underlying disease or concomitant medication. The overall incidence rates of adverse experiences in these trials were similar for tramadol hydrochloride and the active control groups, acetaminophen 300 mg with codeine phosphate 30 mg, and aspirin 325 mg with codeine phosphate 30 mg however the rates of withdrawals due to adverse events appeared to be higher in the tramadol hydrochloride groups.
Table 2
Cumulative Incidence of Adverse Reactions for Tramadol Hydrochloride in Chronic Trials of Nonmalignant Pain (N=427)
| Up to 7 Days | Up to 30 Days | Up to 90 Days | |
| Dizziness/Vertigo | 26% | 31% | 33% |
| Nausea | 24% | 34% | 40% |
| Constipation | 24% | 38% | 46% |
| Headache | 18% | 26% | 32% |
| Somnolence | 16% | 23% | 25% |
| Vomiting | 9% | 13% | 17% |
| Pruritus | 8% | 10% | 11% |
| ‘CNS Stimulation’1 | 7% | 11% | 14% |
| Asthenia | 6% | 11% | 12% |
| Sweating | 6% | 7% | 9% |
| Dyspepsia | 5% | 9% | 13% |
| Dry Mouth | 5% | 9% | 10% |
| Diarrhea | 5% | 9% | 10% |
DRUG ABUSE AND DEPENDENCE Tramadol hydrochloride may induce psychic and physical dependence of the morphine-type (µ-opioid) (See WARNINGS). Dependence and abuse, including drug-seeking behavior and taking illicit actions to obtain the drug are not limited to those patients with prior history of opioid dependence. The risk in patients with substance abuse has been observed to be higher. Tramadol hydrochloride is associated with craving and tolerance development. Withdrawal symptoms may occur if tramadol hydrochloride is discontinued abruptly. These symptoms may include: anxiety, sweating, insomnia, rigors, pain, nausea, tremors, diarrhea, upper respiratory symptoms, piloerection, and rarely hallucinations. Other symptoms that have been seen less frequently with tramadol hydrochloride discontinuation include: panic attacks, severe anxiety, and paresthesias. Clinical experience suggests that withdrawal symptoms may be relieved by reinstitution of opioid therapy followed by a gradual, tapered dose reduction of the medication combined with symptomatic support.
OVERDOSAGE Serious potential consequences of overdosage are respiratory depression, lethargy, coma, seizure, cardiac arrest and death (See WARNINGS). Fatalities have been reported in post marketing in association with both intentional and unintentional overdose with tramadol hydrochloride. In treating an overdose, primary attention should be given to maintaining adequate ventilation along with general supportive treatment. While naloxone will reverse some, but not all, symptoms caused by overdosage with tramadol hydrochloride the risk of seizures is also increased with naloxone administration. In animals convulsions following the administration of toxic doses of tramadol could be suppressed with barbiturates or benzodiazepines but were increased with naloxone. Naloxone administration did not change the lethality of an overdose in mice. Hemodialysis is not expected to be helpful in an overdose because it removes less than 7% of the administered dose in a 4-hour dialysis period.
DOSAGE AND ADMINISTRATION Adults (17 years of age and over) For patients with moderate to moderately severe chronic pain not requiring rapid onset of analgesic effect, the tolerability of tramadol hydrochloride can be improved by initiating therapy with a titration regimen: The total daily dose may be increased by 50 mg as tolerated every 3 days to reach 200 mg/day (50 mg q.i.d.). After titration, tramadol hydrochloride 50 mg to 100 mg can be administered as needed for pain relief every four to six hours, not to exceed 400 mg per day. For the subset of patients for whom rapid onset of analgesic effect is required and for whom the benefits outweigh the risk of discontinuation due to adverse events associated with higher initial doses, tramadol hydrochloride 50 mg to 100 mg can be administered as needed for pain relief every four to six hours, not to exceed 400 mg per day. Individualization of Dose Good pain management practice dictates that the dose be individualized according to patient need using the lowest beneficial dose. Studies with tramadol in adults have shown that starting at the lowest possible dose and titrating upwards will result in fewer discontinuations and increased tolerability. • In all patients with creatinine clearance less than 30 mL/min, it is recommended that the dosing interval of tramadol hydrochloride be increased to 12 hours, with a maximum daily dose of 200 mg. Since only 7% of an administered dose is removed by hemodialysis, dialysis patients can receive their regular dose on the day of dialysis. • The recommended dose for adult patients with cirrhosis is 50 mg every 12 hours. • In general, dose selection for an elderly patient over 65 years old should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy. For elderly patients over 75 years old, total dose should not exceed 300 mg/day.
HOW SUPPLIED Tramadol hydrochloride tablets 50 mg are supplied as unscored, white, round film coated tablets debossed "AN" over “627”. They are supplied as follows: Bottles of 100 (NDC 65162-627-10) Bottles of 500 (NDC 65162-627-50) Bottles of 1000 (NDC 65162-627-11) Store at 20 to 25°C (68 to 77°F). [see USP Controlled Room Temperature] Dispense in a tight container as defined in the USP. Manufactured by: Amneal Pharmaceuticals of NY Hauppauge, NY 11788 Rev. 09-2008
Distributed by: Amneal Pharmaceuticals Glasgow, KY 42141
A Convenience Packed Medical Food And Drug Theratramadol-90 PHYSICIAN THERAPEUTICS Theramine 90 Capsules Tramadol 50 mg 60 Tablets No Refills Without Physician Rx Only NDC# 68405-8038-36 of this co-pack For the Dietary Management of pain and Inflammation Two capsules twice daily or as directed by physician. See product label and insert Theramine Medical Food As prescribed by physician. See product label and product information insert. Tramadol 50 mg Rx Drug Physician Therapeutics LLC Los Angeles, CA 90077 on November 21, 2006


Theratramadol-90TRAMADOL HYDROCHLORIDE, .GAMMA.-AMINOBUTYRIC ACID KIT
| ||||||||||||||||||||||||||||||||||||||||