Thioridazine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- THIORIDAZINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- THIORIDAZINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- THIORIDAZINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
THIORIDAZINE HYDROCHLORIDE DESCRIPTION
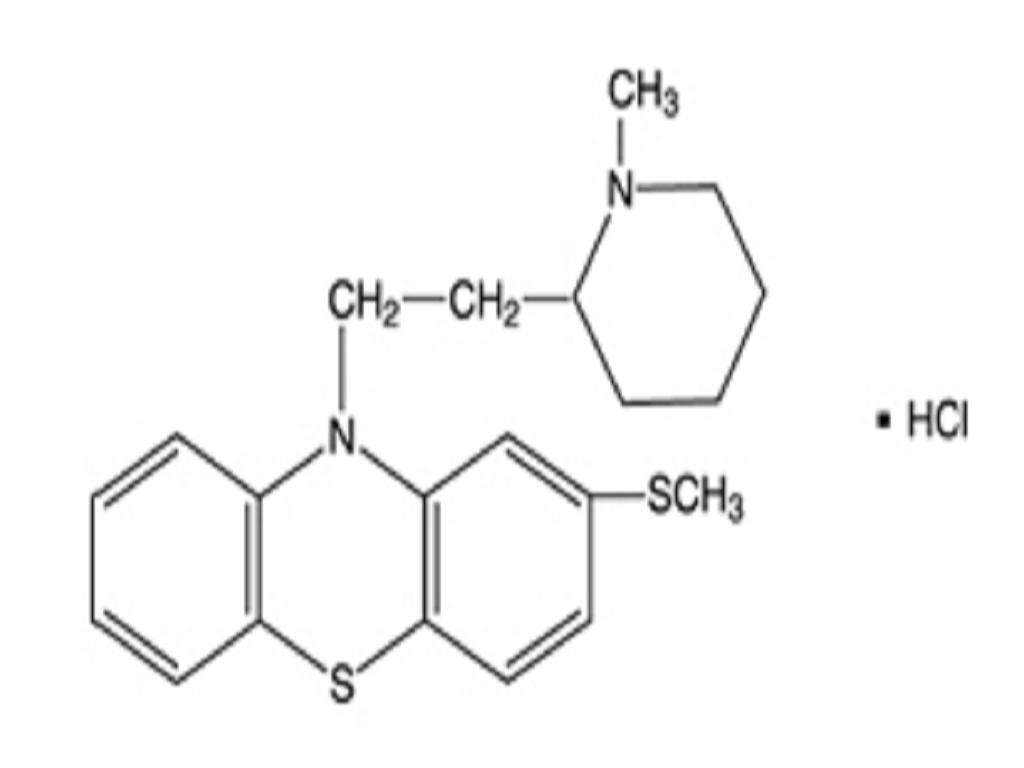
CLINICAL PHARMACOLOGY
WARNINGSCONTRAINDICATIONS
INDICATIONS & USAGE
WARNINGSCONTRAINDICATIONSTHIORIDAZINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGSPRECAUTIONS
WARNINGS
WARNINGS
Increased Mortality in Elderly Patients with Dementia-Related PsychosisBOXED WARNING
Potential for Proarrhythmic Effects
CONTRAINDICATIONSPRECAUTIONS
Tardive Dyskinesia
Information for PatientsADVERSE REACTIONS
Pregnancy
Nonteratogenic Effects
Neuroleptic Malignant Syndrome (NMS)
Central Nervous System Depressants
PRECAUTIONS
Drug Interactions
WARNINGSCONTRAINDICATIONS
Drugs That Inhibit Cytochrome P450 2D6
Drugs That Reduce the Clearance of Thioridazine Through Other Mechanisms
Fluvoxamine
Propranolol
Pindolol
Drugs That Prolong the QTc Interval
Information for Patients
Pediatric Use
DOSAGE AND ADMINISTRATION: Pediatric Patients
Leukopenia, Neutropenia and Agranulocytosis
THIORIDAZINE HYDROCHLORIDE ADVERSE REACTIONS
Central Nervous System
Autonomic Nervous System
Endocrine System
Skin
Cardiovascular System
WARNINGSPhenothiazine Derivatives: Cardiovascular Effects
Other
Post Introduction Reports
Phenothiazine Derivatives
WARNINGS
WARNINGS
WARNINGS
OVERDOSAGE
ADVERSE REACTIONSSigns and Symptoms
Treatment
WARNINGSCONTRAINDICATIONS
1
1
DOSAGE & ADMINISTRATION
INDICATIONSWARNINGSAdults
Pediatric Patients
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

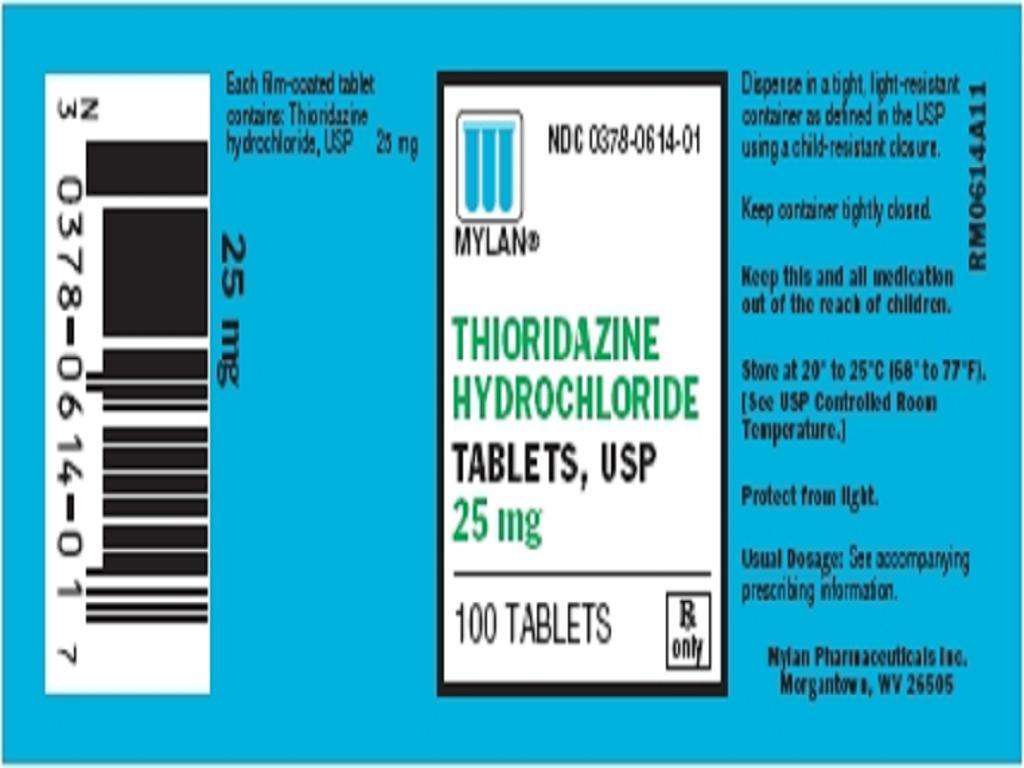
Thioridazine HydrochlorideThioridazine Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!