Thymoglobulin
Sterile Lyophilized Powder For Intravenous Use Only Rx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING
- THYMOGLOBULIN DESCRIPTION
- PHARMACOLOGY
- THYMOGLOBULIN INDICATIONS AND USAGE
- THYMOGLOBULIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- THYMOGLOBULIN ADVERSE REACTIONS
- OVERDOSAGE
- THYMOGLOBULIN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- Package Label - Principal Display Panel – 25 mg Carton
FULL PRESCRIBING INFORMATION
WARNING
Thymoglobulin® should only be used by physicians experienced in immunosuppressive therapy for the management of renal transplant patients.
THYMOGLOBULIN DESCRIPTION
Thymoglobulin® [Anti-thymocyte Globulin (Rabbit)] is a purified, pasteurized, gamma immune globulin, obtained by immunization of rabbits with human thymocytes. This immunosuppressive product contains cytotoxic antibodies directed against antigens expressed on human T-lymphocytes.
Thymoglobulin is a sterile, freeze-dried product for intravenous administration after reconstitution with Sterile Water for Injection, USP (SWFI).
Each 10 mL vial contains 25 mg anti-thymocyte globulin (rabbit) as well as 50 mg glycine, 50 mg mannitol, and 10 mg sodium chloride.
After reconstitution with 5 mL SWFI, each vial of reconstituted product contains approximately 5 mg/mL of Thymoglobulin, of which >90% is rabbit gamma immune globulin (IgG). The reconstituted solution has a pH of 6.5 - 7.2. Human red blood cells are used in the manufacturing process to deplete cross-reactive antibodies to non-T-cell antigens. The manufacturing process is validated to remove or inactivate potential exogenous viruses. All human red blood cells are from US registered or FDA licensed blood banks. A viral inactivation step (pasteurization, i.e., heat treatment of active ingredient at 60°C/10 hr) is performed for each lot. Each Thymoglobulin lot is released following potency testing (lymphocytotoxicity and E-rosette inhibition assays), and cross-reactive antibody testing (hemagglutination, platelet agglutination, anti-human serum protein antibody, antiglomerular basement membrane antibody, and fibroblast toxicity assays on every fifth lot).
PHARMACOLOGY
Mechanism of Action
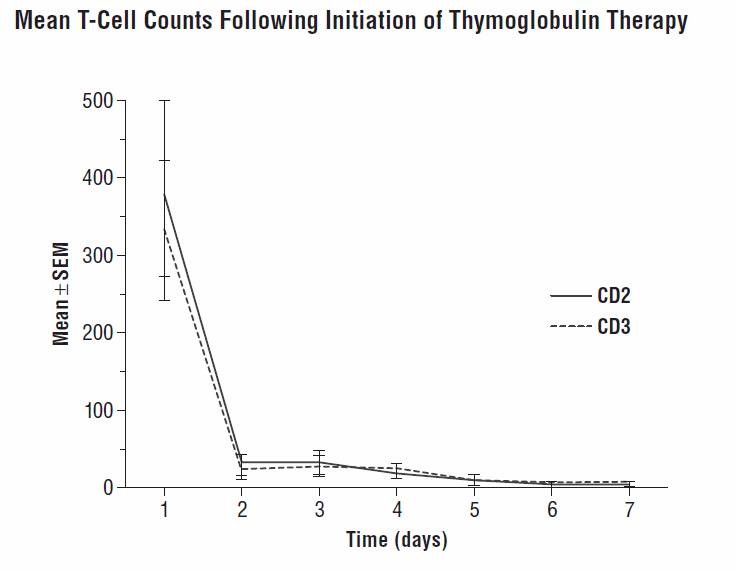
The mechanism of action by which polyclonal antilymphocyte preparations suppress immune responses is not fully understood. Possible mechanisms by which Thymoglobulin may induce immunosuppression in vivo include: T-cell clearance from the circulation and modulation of T-cell activation, homing, and cytotoxic activities. Thymoglobulin includes antibodies against T-cell markers such as CD2, CD3, CD4, CD8, CD11a, CD18, CD25, CD44, CD45, HLA-DR, HLA Class I heavy chains, and ß2 micro-globulin. In vitro, Thymoglobulin (concentrations >0.1 mg/mL) mediates T-cell suppressive effects via inhibition of proliferative responses to several mitogens. In patients, T-cell depletion is usually observed within a day from initiating Thymoglobulin therapy. Thymoglobulin has not been shown to be effective for treating antibody (humoral) mediated rejections.
Pharmacokinetics and Immunogenicity
After an intravenous dose of 1.25 to 1.5 mg/kg/day (over 4 hours for 7-11
days) 4-8 hours post-infusion, Thymoglobulin levels were on average 21.5 mcg/mL
(10-40 mcg/mL) with a half-life of 2-3 days after the first dose, and 87 mcg/mL
(23-170 mcg/mL) after the last dose. During the Thymoglobulin
Clinical Trials
US Phase 3 Study
A controlled, double-blind, multicenter, randomized clinical trial comparing Thymoglobulin and Atgam was conducted at 28 US transplant centers in renal transplant patients (n=163) with biopsy-proven Banff Grade II (moderate), Grade III (severe), or steroid-resistant Grade I (mild) acute graft rejection. This clinical trial rejected the null hypothesis that Thymoglobulin was more than 20% less effective in reversing acute rejection than Atgam. The overall weighted estimate of the treatment difference (Thymoglobulin – Atgam success rate) was 11.1% with a lower 95% confidence bound of 0.07%. Therefore, Thymoglobulin was at least as effective as Atgam in reversing acute rejection episodes.
In the study, patients were randomized to receive 7 to 14 days of Thymoglobulin (1.5 mg/kg/day) or Atgam (15 mg/kg/day). For the entire study, the two treatment groups were comparable with respect to donor and recipient characteristics. During the trial, the FDA approved new maintenance immunosuppressive agents (tacrolimus and mycophenolate). Off-protocol use of these agents occurred during the second half of the study in some patients without affecting the overall conclusions (Thymoglobulin 22/43, Atgam 20/37; p=0.826). The results, however, are presented for the first and second halves of the study (Table 1). In Table 1, successful treatment is presented as those patients whose serum creatinine levels (14 days from the diagnosis of rejection) returned to baseline and whose graft was functioning on day 30 after the end of therapy.
| Sucess/n | Total | First Half | Second Half | |||
|---|---|---|---|---|---|---|
| Thymoglobulin | Atgam | Thymoglobulin | Atgam | Thymglobulin | Atgam | |
| Risk Factor: | ||||||
| Baseline | ||||||
| Rejection Severity: | ||||||
| Mild | 9/10 (90.0%) | 5/8 (62.5%) | 5/5 (100%) | 1/3 (33.3%) | 4/5 (80.0%) | 4/5 (80%) |
| Moderate | 44/58 (75.5%) | 41/58 (70.7%) | 22/26 (84.6%) | 22/32 (68.8%) | 22/32 (68.8%) | 19/26 (73.1%) |
| Severe | 11/14 (71.6%) | 8/14 (57.1%) | 6/8 (75.0%) | 3/8 (37.5%) | 5/6 (83.3%) | 5/6 (83.3%) |
| Overall | 64/82 (78.0%) | 54/80 (67.5%) | 33/39 (84.6%) | 26/43 (60.5%) | 31/43 (72.1%) | 28/37 (75.7%) |
|
Weighted estimate of difference |
11.1% |
19.3% | -3.2% | |||
| Lower one-sided 95% confidence bound | 0.07% | 4.6% | -19.7% | |||
| p Value |
0.061 |
0.008 |
0.625 |
|||
There were no significant differences between the two treatments with respect to (i) day 30 serum creatinine levels relative to baseline, (ii) improvement rate in post-treatment histology, (iii) one-year post-rejection Kaplan-Meier patient survival (Thymoglobulin 93%, n=82 and Atgam 96%, n=80), (iv) day 30 and (v) one-year post-rejection graft survival (Thymoglobulin 83%, n=82; Atgam 75%, n=80).
THYMOGLOBULIN INDICATIONS AND USAGE
Thymoglobulin is indicated for the treatment of renal transplant acute rejection in conjunction with concomitant immunosuppression.
THYMOGLOBULIN CONTRAINDICATIONS
Thymoglobulin is contraindicated in patients with history of allergy or anaphylaxis to rabbit proteins or to any product excipients, or who have active acute or chronic infections which contraindicate any additional immunosuppression.
WARNINGS
Thymoglobulin should only be used by physicians experienced in immunosuppressive therapy for the treatment of renal transplant patients. Medical surveillance is required during Thymoglobulin infusion.
Immune-mediated reactions
Serious immune-mediated reactions have been reported with the use of Thymoglobulin and consist of anaphylaxis or severe cytokine release syndrome (CRS).
Fatal anaphylaxis has been reported. If an anaphylactic reaction occurs, the infusion should be terminated immediately. Medical personnel should be available to treat patients who experience anaphylaxis. Emergency treatment such as 0.3 mL to 0.5 mL aqueous epinephrine (1:1000 dilution) subcutaneously and other resuscitative measures including oxygen, intravenous fluids, antihistamines, corticosteroids, pressor amines, and airway management, as clinically indicated, should be provided. Any further administration of Thymoglobulin to a patient who has a history of anaphylaxis to Thymoglobulin is not recommended.
Severe, acute infusion-associated reactions (IARs) are consistent with CRS which is attributed to the release of cytokines by activated monocytes and lymphocytes. Severe acute CRS can cause serious cardiorespiratory events and/or death (See PRECAUTIONS and ADVERSE REACTIONS: Post-Marketing Experience ).
Infection
Thymoglobulin is routinely used in combination with other immunosuppressive agents. Infections (bacterial, fungal, viral and protozoal), reactivation of infection (particularly cytomegalovirus [CMV]) and sepsis have been reported after Thymoglobulin administration in combination with multiple immunosuppressive agents. Severe acute reactions can be fatal.
PRECAUTIONS
General
Appropriate dosing for Thymoglobulin is different from dosing for other anti-thymocyte globulin (ATG) products, as protein composition and concentrations vary depending on the source of ATG used. Physicians should therefore exercise care to ensure that the dose prescribed is appropriate for the ATG product being administered.
Thymoglobulin should be used under strict medical supervision in a hospital setting, and patients should be carefully monitored during the infusion. The first dose should be infused over a minimum of 6 hours into a high-flow vein. Close compliance with the recommended dosage and infusion time may reduce the incidence and severity of infusion associated reactions (IARs). Additionally, reducing the infusion rate may minimize many of these IARs. Premedication with corticosteroids, acetaminophen, and/or an antihistamine and/or slowing the infusion rate may reduce reaction incidence and intensity (See DOSAGE AND ADMINISTRATION ).
Rapid infusion rates have been reported with case reports consistent with cytokine release syndrome (CRS). Severe acute CRS can be fatal.
Hematologic Effects
Thrombocytopenia and/or leukopenia (including lymphopenia and neutropenia) have been identified and are reversible following dose adjustments (See DOSAGE AND ADMINISTRATION ).
Infection
Infections, reactivation of infection, and sepsis have been reported after Thymoglobulin administration in combination with multiple immunosuppressive agents. Careful patient monitoring and appropriate anti-infective prophylaxis are recommended.
Malignancy
Use of immunosuppressive agents, including Thymoglobulin, may increase the incidence of malignancies, including lymphoma or post-transplant lymphoproliferative disease (PTLD) (See ADVERSE REACTIONS: Post-Marketing Experience ).
Special Considerations for Thymoglobulin Infusion
Reactions at the infusion site can occur and may include pain, swelling, and erythema.
The recommended route of administration for Thymoglobulin is intravenous infusion using a high-flow vein (See DOSAGE AND ADMINISTRATION ).
Immunizations
The safety of immunization with attenuated live vaccines following Thymoglobulin therapy has not been studied; therefore, immunization with attenuated live vaccines is not recommended for patients who have recently received Thymoglobulin.
Laboratory Tests
During Thymoglobulin therapy, monitoring the lymphocyte count (i.e., total lymphocyte and/or T-cell subset) may help assess the degree of T-cell depletion (See Pharmacokinetics and Immunogenicity ). For safety, WBC and platelet counts should also be monitored (See DOSAGE AND ADMINISTRATION ).
Drug Interactions
- No drug interaction studies have been performed.
- Because Thymoglobulin is administered to patients receiving a standard immunosuppressive regimen, this may predispose patients to overimmunosuppression. Many transplant centers decrease maintenance immunosuppression therapy during the period of antibody therapy.
- Thymoglobulin can stimulate the production of antibodies which crossreact with rabbit immune globulins (See Pharmacokinetics and Immunogenicity ).
Drug/Laboratory Test Interactions
Thymoglobulin has not been shown to interfere with any routine clinical laboratory tests which do not use immunoglobulins. Thymoglobulin may interfere with rabbit antibody-based immunoassays and with cross-match or panel-reactive antibody cytotoxicity assays.
Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic and mutagenic potential of Thymoglobulin and its potential to impair fertility have not been studied.
Pregnancy: Pregnancy Category C
Animal reproduction studies have not been conducted with Thymoglobulin. It is also not known whether Thymoglobulin can cause fetal harm or can affect reproduction capacity. Thymoglobulin should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Thymoglobulin has not been studied in nursing women. It is not known whether this drug is excreted in human milk. Because other immunoglobulins are excreted in human milk, breast-feeding should be discontinued during Thymoglobulin therapy.
Pediatric Use
The safety and effectiveness of Thymoglobulin in pediatric patients has not been established in controlled trials. However, the dose, efficacy, and adverse event profile are not thought to be different from adults based on limited European studies and US compassionate use.
THYMOGLOBULIN ADVERSE REACTIONS
Clinical Trials
US Phase 3 Study
Thymoglobulin adverse events are generally manageable or reversible. In the US Phase 3 controlled clinical trial (n=163) comparing the efficacy and safety of Thymoglobulin and Atgam, there were no significant differences in clinically significant adverse events between the two treatment groups (Table 2). Malignancies were reported in 3 patients who received Thymoglobulin and in 3 patients who received Atgam during the one-year follow-up period. These included two post-transplant lymphoproliferative diseases (PTLDs) in the Thymoglobulin group and two PTLDs in the Atgam group.
| Preferred Term |
Thymoglobulin n=82 |
Atgam n=81 |
p Value |
||
|---|---|---|---|---|---|
| No.
of Patients |
(%) | No.
of Patients |
(%) | ||
| Frequently Reported Events | |||||
| Fever | 52 | (63.4) | 51 | (63.0) | 1.0 |
| Chills | 47 | (57.3) | 35 | (43.2) | 0.086 |
| Leukopenia | 47 | (57.3) | 24 | (29.6) | <0.001 |
| Pain | 38 | (46.3) | 35 | (43.2) | 0.753 |
| Headache | 33 | (40.2) | 28 | (34.6) | 0.518 |
| Abdominal pain | 31 | (37.8) | 22 | (27.2) | 0.181 |
| Diarrhea | 30 | (36.6) | 26 | (32.1) | 0.622 |
| Hypertension | 30 | (36.6) | 23 | (28.4) | 0.316 |
| Nausea | 30 | (36.6) | 23 | (28.4) | 0.316 |
| Thrombocytopenia | 30 | (36.6) | 36 | (44.4) | 0.341 |
| Peripheral edema | 28 | (34.1) | 28 | (34.6) | 1.0 |
| Dyspnea | 23 | (28.0) | 16 | (19.8) | 0.271 |
| Asthenia | 22 | (26.8) | 26 | (32.1) | 0.495 |
| Hyperkalemia | 22 | (26.8) | 15 | (18.5) | 0.262 |
| Tachycardia | 22 | (26.8) | 19 | (23.5) | 0.719 |
|
Significant Events |
|||||
| Leukopenia | 47 | (57.3) | 24 | (29.6) | <0.001 |
| Malaise | 11 | (13.4) | 3 | (3.7) | 0.047 |
| Dizziness | 7 | (8.5) | 20 | (24.7) | 0.006 |
Infections occurring in both treatment groups during the 3-month follow-up are summarized in Table 3. No significant differences were seen between the Thymoglobulin and Atgam groups for all types of infections, and the incidence of cytomegalovirus (CMV) infection was equivalent in both groups. (Viral prophylaxis was by the center’s discretion during antibody treatment, but all centers used gancyclovir infusion during treatment.)
|
BODY SYSTEM Preferred Term |
Thymoglobulin n=82 |
Atgam n=81 |
p Value |
||||
|---|---|---|---|---|---|---|---|
|
No.
of Patients |
(%) |
Total Reports |
No.
of Patients |
(%) | Total Reports |
||
| BODY AS A WHOLE | 30 | (36.6) | 36 | 22 | (27.2) | 29 | 0.240 |
| Infection | 25 | (30.5) | 26 | 19 | (23.5) | 21 | 0.378 |
| Other | 14 | (17.1) | 15 | 11 | (13.6) | 12 | 0.665 |
| CMV | 11 | (13.4) | 11 | 9 | (11.1) | 9 | 0.812 |
| Sepsis | 10 | (12.2) | 10 | 7 | (9.6) | 7 | 0.610 |
| Moniliasis | 0 | (0.0) | 0 | 1 | (1.2) | 1 | 0.497 |
| DIGESTIVE | 5 | (6.1) | 5 | 3 | (3.7) | 3 | 0.720 |
|
Gastrointestinal moniliasis |
4 | (4.9) | 4 | 1 | (1.2) | 1 | 0.367 |
| Oral moniliasis | 3 | (3.7) | 0 | 2 | (2.5) | 1 | 0.497 |
| Gastritis | 1 | (1.2) | 1 | 0 | (0.0) | 0 | 1.000 |
| RESPIRATORY | 0 | (0.0) | 0 | 1 | (1.2) | 1 | 0.497 |
| Pneumonia | 0 | (0.0) | 0 | 1 | (1.2) | 1 | 0.497 |
| SKIN | 4 | (4.9) | 4 | 0 | (0.0) | 0 | 0.120 |
| Herpes simplex | 4 | (4.9) | 4 | 0 | (0.0) | 0 | 0.120 |
| UROGENITAL | 15 | (18.3) | 15 | 22 | (29.2) | 22 | 0.195 |
| Urinary tract infection | 15 | (18.3) | 15 | 21 | (25.9) | 21 | 0.262 |
| Vaginitis | 0 | (0.0) | 0 | 1 | (1.2) | 1 | 0.497 |
| NOT SPECIFIED | 0 | (0.0) | 0 | 2 | (2.5) | 2 | 0.245 |
Post-marketing Experience
The following adverse reactions have been identified during post approval use of Thymoglobulin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Infusion-Associated Reactions and Immune System Disorders
IARs may occur following the administration of Thymoglobulin and may occur as soon as the first or second infusion during a single course of Thymoglobulin treatment. Clinical manifestations of infusion-associated reactions IARs have included some of the following signs and symptoms: fever, chills/rigors, dyspnea, nausea/vomiting, diarrhea, hypotension or hypertension, malaise, rash, and/or headache. IARs with Thymoglobulin are generally manageable with a reduction in infusion rates and/or with medications (See PRECAUTIONS ). Serious and fatal anaphylactic reactions have been reported (See WARNINGS ). The fatalities occurred in patients who did not receive epinephrine during the event.
IARs consistent with cytokine release syndrome (CRS) have been reported. Severe and potentially life-threatening CRS have also been reported. Post-marketing reports of severe CRS have included cardiorespiratory dysfunction (including hypotension, acute respiratory distress syndrome, pulmonary edema, myocardial infarction, tachycardia, and/or death).
During post-marketing surveillance, reactions such as fever, rash, arthralgia, and/or myalgia, indicating possible serum sickness, have been reported. Serum sickness tends to occur 5 to 15 days after onset of Thymoglobulin therapy. Symptoms are manageable with corticosteroid treatment.
Adverse Events Due to Immunosuppression
Infections, reactivation of infection, and sepsis have been reported after Thymoglobulin administration in combination with multiple immunosuppressive agents (See WARNINGS and PRECAUTIONS ). Malignancies including, but not limited to post-transplant lymphoproliferative disorder (PTLD) and other lymphomas as well as solid tumors have been reported (See PRECAUTIONS ). These adverse events were reported with use of a combination of multiple immunosuppressive agents.
OVERDOSAGE
Thymoglobulin overdosage may result in leukopenia (including lymphopenia and neutropenia) or thrombocytopenia, which can be managed with dose reduction (See DOSAGE AND ADMINISTRATION ).
THYMOGLOBULIN DOSAGE AND ADMINISTRATION
The recommended dosage of Thymoglobulin for treatment of acute renal graft rejection is 1.5 mg/kg of body weight administered daily for 7 to 14 days. The recommended route of administration is intravenous infusion using a high-flow vein. Thymoglobulin should be infused over a minimum of 6 hours for the first infusion and over at least 4 hours on subsequent days of therapy.
Thymoglobulin should be administered through an in-line 0.22 micrometer filter.
Thymoglobulin is supplied as a 10 mL vial containing lyophilized (solid) Thymoglobulin (25 mg).
Please see Preparation for Administration for vial reconstitution and dilution in infusion solution recommendations. Investigations indicate that Thymoglobulin is less likely to produce side effects when administered at the recommended flow rate. Administration of antiviral prophylactic therapy is recommended. Premedication with corticosteroids, acetaminophen, and/or an antihistamine 1 hour prior to the infusion is recommended and may reduce the incidence and intensity of side effects during the infusion (See PRECAUTIONS: General and ADVERSE REACTIONS: Post-Marketing Experience ). Medical personnel should monitor patients for adverse events during and after infusion. Monitoring T-cell counts (absolute and/or subsets) to assess the level of T-cell depletion is recommended. Total white blood cell and platelet counts should be monitored.
Overdosage of Thymoglobulin may result in leukopenia (including lymphopenia and neutropenia) and/or thrombocytopenia. The Thymoglobulin dose should be reduced by one-half if the WBC count is between 2,000 and 3,000 cells/mm3 or if the platelet count is between 50,000 and 75,000 cells/mm3. Stopping Thymoglobulin treatment should be considered if the WBC count falls below 2,000 cells/mm3 or platelets below 50,000 cells/mm3.
Preparation for Administration
Reconstitution
After calculating the number of vials needed, using aseptic technique,
reconstitute each vial of Thymoglobulin with 5 mL of Sterile Water for Injection,
USP (SWFI). Reconstituted Thymoglobulin is physically and chemically stable for up
to 24 hours at room temperature; however, room temperature storage is not
recommended. As Thymoglobulin contains no preservatives, reconstituted product
should be used immediately.
- Allow Thymoglobulin vials to reach room temperature before reconstituting the lyophilized product.
- Aseptically remove caps to expose rubber stoppers.
- Clean stoppers with germicidal or alcohol swab.
- Aseptically reconstitute each vial of Thymoglobulin lyophilized powder with the 5 mL of SWFI.
- Rotate vial gently until powder is completely dissolved. Each reconstituted vial contains 25 mg or 5 mg/mL of Thymoglobulin.
- Inspect solution for particulate matter after reconstitution. Should some particulate matter remain, continue to gently rotate the vial until no particulate matter is visible. If particulate matter persists, discard this vial.
Dilution
- Transfer the contents of the calculated number of Thymoglobulin vials into the bag of infusion solution (saline or dextrose). Recommended volume: per one vial of Thymoglobulin use 50 mL of infusion solution (total volume usually between 50 to 500 mL).
- Mix the solution by inverting the bag gently only once or twice.
Infusion
- Follow the manufacturer’s instructions for the infusion administration set. Infuse through a 0.22 micrometer filter into a high-flow vein.
- Set the flow rate to deliver the dose over a minimum of 6 hours for the first dose and over at least 4 hours for subsequent doses.
HOW SUPPLIED
Thymoglobulin is available as sterile, lyophilized powder to be reconstituted with sterile Water for Injection, USP (SWFI). Each package contains a 10 mL vial of freeze-dried Thymoglobulin (25 mg) NDC# 58468-0080-1.
Storage
- Store in refrigerator at 2°C to 8°C (36°F to 46°F).
- Protect from light.
- Do not freeze.
- Do not use after the expiration date indicated on the label.
- Reconstituted Thymoglobulin is physically and chemically stable for up to 24 hours at room temperature; however, room temperature storage is not recommended. As Thymoglobulin contains no preservatives, reconstituted product should be used immediately.
- Infusion solutions of Thymoglobulin must be used immediately.
- Any unused drug remaining after infusion must be discarded.
REFERENCES
- Bonnefoy-Bérard N, et al. Antibodies against functional leukocyte surface molecules in polyclonal antilymphocyte and antithymocyte globulins. Transplantation (1991) 51 :669-673.
- Bonnefoy-Bérard N, et al. Inhibition of CD25 (IL-2Rα) expression and Tcell proliferation by polyclonal anti-thymocyte globulins. Immunology (1992) 77 :61-67.
- Bonnefoy-Bérard N, et al. Antiproliferative effect of antilymphocyte globulins on B cells and B-cell lines. Blood (1992) 79 :2164-2170.
- Bonnefoy-Bérard N, Revillard J-P. Mechanisms of immunosuppression induced by antilymphocyte globulins and OKT3. J Heart Lung Transplant (1996) 15 :435-442.
- Bourdage J, et al. Comparative polyclonal antithymocyte globulin and antilymphocyte/antilymphoblast globulin anti-CD antigen analysis by flow cytometry. Transplantation (1995) 59 :1194-1200.
- Broyer M, et al. Triple therapy including cyclosporine A versus conventional regimen–a randomized prospective study in pediatric kidney transplantation. Transplant Proc (1987) 19 :3582-3585.
- Clark KR, et al. Administration of ATG according to the absolute T lymphocyte count during therapy for steroid-resistant rejection. Transpl Int (1993) 6 :18-21.
- Gaber AO, et al. Results of the double-blind, randomized, multicenter, phase III clinical trial of Thymoglobulin versus Atgam in the treatment of acute graft rejection episodes after renal transplantation. Transplantation (1998) 66 :29-37.
- Guttmann RD, et al. Pharmacokinetics, foreign protein immune response, cytokine release, and lymphocyte subsets in patients receiving Thymoglobuline and immunosuppression. Transplant Proc (1997) 29 (suppl 7A):24S-26S.
- Ippoliti G, et al. Prophylactic use of rabbit ATG vs horse ALG in hearttransplanted patients under Sandimmun (CyA) therapy: clinical and immunological effects. Clin Transplantation (1989) 3 :204-208.
Manufactured
for:
By:
Genzyme
Corporation
Genzyme Polyclonals, S.A.S.
500 Kendall
Street
Marcy L’Etoile, France
Cambridge, MA 02142
USA
US License No. 1596
©2007 Genzyme Corporation. All rights reserved.
Package Label - Principal Display Panel – 25 mg Carton
NDC 58468-0080-1
Thymoglobulin®
Anti-thymocyte Globulin (Rabbit)
25 mg
For Intravenous Infusion Only
Rx Only
genzyme

ThymoglobulinAnti-thymocyte Globulin (Rabbit) INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||