Thyrogen
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use THYROGEN safely and effectively. See full prescribing information for THYROGEN. THYROGEN® (thyrotropin alfa for injection), for intramuscular injection Initial U.S. Approval: 1998INDICATIONS AND USAGETHYROGEN® is a thyroid stimulating hormone indicated for: Diagnostic: Use as an adjunctive diagnostic tool for serum thyroglobulin (Tg) testing with or without radioiodine imaging in the follow-up of patients with well-differentiated thyroid cancer who have previously undergone thyroidectomy. ( 1.1 ) Limitations of Use: THYROGEN-stimulated Tg levels are generally lower than, and do not correlate with Tg levels after thyroid hormone withdrawal. Even when THYROGEN-Tg testing is performed in combination with radioiodine imaging , there remains a risk of missing a diagnosis of thyroid cancer or underestimating the extent of the disease. Anti-Tg Antibodies may confound the Tg assay and render Tg levels uninterpretable. Ablation: Use as an adjunctive treatment for radioiodine ablation of thyroid tissue remnants in patients who have undergone a near-total or total thyroidectomy for well-differentiated thyroid cancer and who do not have evidence of distant metastatic thyroid cancer. ( 1.2 ) Limitations of Use : The effect of THYROGEN on long term thyroid cancer outcomes has not been determined. DOSAGE AND ADMINISTRATION THYROGEN should be used by physicians knowledgeable in the management of patients with thyroid cancer. ( 2.1 ) A two-injection regimen is recommended: THYROGEN 0.9 mg is administered intramuscularly, followed by a second 0.9 mg intramuscular injection 24 hours later. ( 2.1 ) DOSAGE FORMS AND STRENGTHSLyophilized powder containing 1.1 mg of thyrotropin alfa for single use after reconstitution with Sterile Water for Injection. ( 3 )CONTRAINDICATIONSNone ( 4 )WARNINGS AND PRECAUTIONS Risk of THYROGEN-induced hyperthyroidism. Hospitalization for administration of THYROGEN and post administrative observation should be considered for patients at risk.( 5.1 ) Stroke in female patients as well as other neurologic events in patients with central nervous system metastases. ( 5.2 ) ( 5.3 ) Sudden, rapid and painful enlargement in distant metastatic thyroid cancer ( 5.3 ) Side EffectsThe most common adverse reactions reported in clinical trials were nausea and headache. ( 6.1 ) To report SUSPECTED ADVERSE REACTIONS, contact Genzyme Corporation at 800-745-4447 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. USE IN SPECIFIC POPULATIONSRenal Impairment: Elimination of THYROGEN is significantly slower in dialysis-dependent end stage renal disease patients, resulting in prolonged elevation of TSH levels. ( 8.6 )
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 THYROGEN INDICATIONS AND USAGE
- 2 THYROGEN DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 THYROGEN CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 THYROGEN ADVERSE REACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 THYROGEN DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Adjunctive Diagnostic Tool for Serum Thyroglobulin Testing in Well Differentiated Thyroid Cancer
THYROGEN® is indicated for use as an adjunctive diagnostic tool for serum thyroglobulin (Tg) testing with or without radioiodine imaging in the follow-up of patients with well-differentiated thyroid cancer who have previously undergone thyroidectomy.
Limitations of Use:
- THYROGEN-stimulated Tg levels are generally lower than, and do not correlate with, Tg levels after thyroid hormone withdrawal [ see Clinical Studies ( 14.1 )].
- Even when THYROGEN-stimulated Tg testing is performed in combination with radioiodine imaging, there remains a risk of missing a diagnosis of thyroid cancer or of underestimating the extent of disease.
- Anti-Tg antibodies may confound the Tg assay and render Tg levels uninterpretable [ see Clinical Studies ( 14.1 )]. Therefore, in such cases, even with a negative or low-stage THYROGEN radioiodine scan, consideration should be given to further evaluating patients.
1.2 Adjunct to Treatment for Ablation in Well Differentiated Thyroid Cancer
THYROGEN is indicated for use as an adjunctive treatment for radioiodine ablation of thyroid tissue remnants in patients who have undergone a near-total or total thyroidectomy for well-differentiated thyroid cancer and who do not have evidence of distant metastatic thyroid cancer.
Limitations of Use :
- The effect of THYROGEN on long-term thyroid cancer outcomes has not been determined. Due to the relatively small clinical experience with THYROGEN in remnant ablation, it is not possible to conclude whether long-term thyroid cancer outcomes would be equivalent after use of THYROGEN or use of thyroid hormone withholding for TSH elevation prior to remnant ablation.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
THYROGEN should be used by physicians knowledgeable in the management of patients with thyroid cancer.
THYROGEN is indicated as a two-injection regimen. The recommended dosage of THYROGEN is a 0.9 mg intramuscular injection to the buttock followed by a second 0.9 mg intramuscular injection to the buttock 24 hours later.
THYROGEN should be administered intramuscularly only. THYROGEN should not be administered intravenously.
Pretreatment with glucocorticoids should be considered for patients in whom tumor expansion may compromise vital anatomic structures [ see Warnings and Precautions ( 5.3 )].
Routine measurement of serum TSH levels is not recommended after THYROGEN use.
2.2 Reconstitution, Preparation, and Administration of THYROGEN
The supplied lyophilized powder must be reconstituted with Sterile Water for Injection. THYROGEN should be prepared, and administered in the following manner:
- Add 1.2 mL of Sterile Water for Injection to the vial containing the THYROGEN lyophilized powder.
- Swirl the contents of the vial until all the material is dissolved. Do not shake the solution. The reconstituted THYROGEN solution has a concentration of 0.9 mg of thyrotropin alfa per mL.
- Visually inspect the reconstituted solution for particulate matter and discoloration prior to administration. The reconstituted THYROGEN solution should be clear and colorless. Do not use if the solution has particulate matter or is cloudy or discolored.
- Withdraw 1 mL of the reconstituted THYROGEN solution (0.9 mg of thyrotropin alfa) and inject intramuscularly in the buttocks.
- The reconstituted THYROGEN solution must be injected within 3 hours unless refrigerated; if refrigerated, the reconstituted solution may be kept for up to 24 hours.
- Discard unused portions. Do not mix with other substances.
2.3 Timing of Serum Thyroglobulin Testing Following THYROGEN Administration
For serum thyroglobulin testing, the serum sample should be obtained 72 hours after the final injection of THYROGEN [ see Clinical Studies ( 14.1 ) ].
2.4 Timing for Remnant Ablation and Diagnostic Scanning Following THYROGEN Administration
Oral radioiodine should be given 24 hours after the second injection of THYROGEN in both remnant ablation and diagnostic scanning. The activity of 131I is carefully selected at the discretion of the nuclear medicine physician.
Diagnostic scanning should be performed 48 hours after the radioiodine administration.
3 DOSAGE FORMS AND STRENGTHS
THYROGEN is a lyophilized powder containing 1.1 mg of thyrotropin alfa for single use after reconstitution with Sterile Water for Injection.
Supplied as:
- Two vial kit (two vials of lyophilized thyrotropin alfa).
- Four vial kit (two vials of lyophilized thyrotropin alfa) and two vials of 10 mL vials of Sterile Water for Injection).
4 CONTRAINDICATIONS
None
5 WARNINGS AND PRECAUTIONS
5.1 THYROGEN-induced Hyperthyroidism
When given to patients who have substantial thyroid tissue still in situ or functional thyroid cancer metastases, THYROGEN is known to cause a transient (over 7 to 14 days) but significant rise in serum thyroid hormone concentration. There have been reports of death in non-thyroidectomized patients and in patients with distant metastatic thyroid cancer in which events leading to death occurred within 24 hours after administration of THYROGEN. Patients with residual thyroid tissue at risk for THYROGEN-induced hyperthyroidism include the elderly and those with a known history of heart disease. Hospitalization for administration of THYROGEN and post-administration observation in patients at risk should be considered.
5.2 Stroke
There are postmarketing reports of radiologically-confirmed stroke and neurological findings suggestive of stroke unconfirmed radiologically (e.g., unilateral weakness) occurring within 72 hours (range 20 minutes to three days) of THYROGEN administration in patients without known central nervous system metastases. The majority of such patients were young women taking oral contraceptives at the time of their event or had other risk factors for stroke, such as smoking or a history of migraine headaches. The relationship between THYROGEN administration and stroke is unknown. Patients should be well-hydrated prior to treatment with THYROGEN.
5.3 Sudden Rapid Tumor Enlargement
Sudden, rapid and painful enlargement of residual thyroid tissue or distant metastases can occur following treatment with THYROGEN. This may lead to acute symptoms, which depend on the anatomical location of the tissue. Such symptoms include acute hemiplegia, hemiparesis, and loss of vision one to three days after THYROGEN administration. Laryngeal edema, pain at the site of distant metastasis, and respiratory distress requiring tracheotomy have also been reported after THYROGEN administration.
Pretreatment with glucocorticoids should be considered for patients in whom tumor expansion may compromise vital anatomic structures.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to THYROGEN in 481 thyroid cancer patients who participated in a total of 6 clinical trials of THYROGEN: 4 trials for diagnostic use and 2 trials for ablation. In clinical trials, patients had undergone near-total thyroidectomy and had a mean age of 46.1 years. Thyroid cancer diagnosis was as follows: papillary (69.2%), follicular (12.9%), Hurthle cell (2.3%) and papillary/follicular 15.6%. Most patients received 2 intramuscular injections of 0.9 mg of THYROGEN injection given 24 hours apart [ see Clinical Studies ( 14.1 ) ( 14.2 ) ].
The safety profile of patients who have undergone thyroidectomy and received THYROGEN as adjunctive treatment for radioiodine ablation of thyroid tissue remnants for well-differentiated thyroid cancer did not differ from that of patients who received THYROGEN for diagnostic purposes.
Reactions reported in ≥ 1% of patients in the combined trials are summarized in Table 1. In some studies, an individual patient may have participated in both THYROGEN and thyroid hormone withdrawal [ see Clinical Studies ( 14.1 ) ( 14.2 ) ].
Table 1: Summary of Adverse Reactions by THYROGEN and Thyroid Hormone Withdrawal in Pooled Clinical Trials (≥1% of Patients in any Phase)
|
Preferred Term |
THYROGEN (N=481) n (%) |
Thyroid Hormone Withdrawal (N=418) n (%) |
|
Nausea |
53 (11) |
2 (<1) |
|
Headache |
29 (6) |
0 |
|
Fatigue |
11 (2) |
2 (<1) |
|
Vomiting |
11 (2) |
0 |
|
Dizziness |
9 (2) |
0 (0.0) |
|
Asthenia |
5 (1) |
1 (<1) |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of THYROGEN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Transient (<48 hours) influenza-like symptoms, including fever (>100°F/38°C), chills/shivering, myalgia/arthralgia, fatigue/asthenia/malaise, headache, and chills.
- Hypersensitivity including urticaria, rash, pruritus, flushing, and respiratory signs and symptoms.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with THYROGEN.
It is also not known whether THYROGEN can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. THYROGEN should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether the drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when THYROGEN is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
In pooled clinical studies of THYROGEN, 60 patients (12%) were >65 years, and 421 (88%) were ≤ 65 years of age. Results from controlled trials do not indicate a difference in the safety and efficacy of THYROGEN between adult patients less than 65 years and those over 65 years of age [ see Warnings and Precautions ( 5.1 )].
8.6 Renal Impairment
Elimination of THYROGEN is significantly slower in dialysis-dependent end stage renal disease (ESRD) patients, resulting in prolonged elevation of TSH levels.
10 OVERDOSAGE
In clinical trials of THYROGEN, three patients experienced symptoms after receiving THYROGEN doses higher than those recommended. Two patients had nausea after a 2.7 mg IM dose (3 times the recommended dose), and in one of these patients, the event was accompanied by weakness, dizziness and headache. Another patient experienced nausea, vomiting and hot flashes after a 3.6 mg IM dose (4 times the recommended dose). There is no specific therapy for THYROGEN overdose. Supportive care is recommended.
11 DESCRIPTION
Each vial of THYROGEN contains 1.1 mg thyrotropin alfa, 36 mg Mannitol, 5.1 mg Sodium Phosphate, and 2.4 mg Sodium Chloride.
THYROGEN (thyrotropin alfa for injection) contains recombinant human thyroid stimulating hormone (TSH). Thyrotropin alfa is synthesized in a genetically modified Chinese hamster ovary cell line.
Thyrotropin alfa is a heterodimeric glycoprotein comprised of two non-covalently linked subunits, an alpha subunit of 92 amino acid residues containing two N-linked glycosylation sites and a beta subunit of 118 residues containing one N-linked glycosylation site. The amino acid sequence of thyrotropin alfa is identical to that of human pituitary TSH.
Both thyrotropin alfa and naturally occurring human pituitary TSH are synthesized as a mixture of glycosylation variants. Unlike pituitary TSH, which is secreted as a mixture of sialylated and sulfated forms, thyrotropin alfa is sialylated but not sulfated. The biological activity of thyrotropin alfa is determined by a cell-based bioassay. In this assay, cells expressing a functional TSH receptor and a cAMP-responsive element coupled to a heterologous reporter gene, luciferase, enable the measurement of thyrotropin alfa activity by measuring the luciferase response. The specific activity of thyrotropin alfa is determined relative to an internal Genzyme reference standard that was calibrated against the World Health Organization (WHO) human TSH reference standard.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Thyrotropin (TSH) is a pituitary hormone that stimulates the thyroid gland to produce thyroid hormone. Binding of thyrotropin alfa to TSH receptors on normal thyroid epithelial cells or on well-differentiated thyroid cancer tissue stimulates iodine uptake and organification, and synthesis and secretion of thyroglobulin (Tg), triiodothyronine (T3) and thyroxine (T4).
The effect of thyroid stimulating hormone activation of thyroid cells is to increase uptake of radioiodine to allow scan detection or radioiodine killing of thyroid cells. TSH activation also leads to the release of thyroglobulin by thyroid cells. Thyroglobulin functions as a tumor marker which is detected in blood specimens.
12.3 Pharmacokinetics
The pharmacokinetics of THYROGEN were studied in 16 patients with well-differentiated thyroid cancer given a single 0.9 mg IM dose. Mean peak serum TSH concentrations of 116 ± 38 mU/L were reached between 3 and 24 hours after injection (median of 10 hours). The mean apparent elimination half-life was 25 ± 10 hours. The organ(s) of TSH clearance in man have not been identified, but studies of pituitary-derived TSH suggest the involvement of the liver and kidneys.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term toxicity studies in animals have not been performed with THYROGEN to evaluate the carcinogenic potential of the drug. THYROGEN was not mutagenic in the bacterial reverse mutation assay. Studies have not been performed with THYROGEN to evaluate the effects on fertility.
13.2 Animal Pharmacology and/or Toxicology
Four toxicology studies, two in rodents and two in primates, have been conducted using both single and repeated daily injections of thyrotropin alfa. In a single-dose study utilizing male and female rats bolus injections were given at levels up to 7.14 IU/kg (equivalent to 50 times the expected single human dose). There were no effects, either gross or microscopic, in this study which could be attributed to the administration of thyrotropin alfa. In a repeated-dose study, rats were given thyrotropin alfa in the form of 5 daily intramuscular injections at levels up to 1.43 IU/kg (equivalent to 10 times the expected single human dose) without dose-related toxic effects observed.
A single-dose study in male and female cynomolgus monkeys used single intramuscular injections of thyrotropin alfa at levels equivalent to the expected dose and 0.25 and 4 times the expected single human dose. There were no changes that were regarded as indicative of an adverse or toxic response to thyrotropin alfa. Cynomolgus monkeys were also administered thyrotropin alfa as three consecutive daily bolus intramuscular injections at levels extending to 4 times the dose in humans. There were no changes that are considered to indicate an adverse or toxic response to thyrotropin alfa.
14 CLINICAL STUDIES
14.1 Clinical Trials of THYROGEN as an Adjunctive Diagnostic Tool
Two prospective, randomized phase 3 clinical trials were conducted in patients with well-differentiated thyroid cancer to compare 131I whole body scans obtained after THYROGEN injection to 131I whole body scans after thyroid hormone withdrawal. A cross-over, non-blinded design was used in both trials. Oral radioiodine was given 24 hours after the second injection of THYROGEN, and scanning was done 48 hours after the radioiodine administration. Each patient was scanned first following THYROGEN and then scanned after thyroid hormone withdrawal. In both studies, the primary endpoint was the rate of concordant scans (scan findings in agreement in a given patient using each preparation method).
Study 1 (n=127) compared the diagnostic scanning following a THYROGEN regimen of 0.9 mg IM daily on two consecutive days to thyroid hormone withdrawal. In addition to body scans, Study 2 (n=229) also compared thyroglobulin (Tg) levels obtained after THYROGEN to those at baseline and to those after thyroid hormone withdrawal. All Tg testing was performed in a central laboratory using a radioimmunoassay (RIA) with a functional sensitivity of 2.5 ng/mL. Patients who were included in the Tg analysis were those who had undergone total or near-total thyroidectomy with or without 131I ablation, had < 1% uptake in the thyroid bed on a scan after thyroid hormone withdrawal, and did not have detectable anti-Tg antibodies. The maximum THYROGEN Tg value was obtained 72 hours after the final THYROGEN injection, and this value was used in the analysis.
Diagnostic Radioiodine Whole Body Scan Results
Study 1 enrolled 127 patients, 71% were female and 29% male, and mean age was 44 years. The study included the following forms of differentiated thyroid cancer: papillary cancer (88%), follicular cancer (9%), and Hurthle cell (2%). Study results are displayed in Table 2.
In Study 2, patients with differentiated thyroid cancer who had been thyroidectomized (n = 229) were randomized into one of two THYROGEN treatment regimens: THYROGEN 0.9 mg IM daily on two consecutive days (n = 117), and THYROGEN 0.9 mg IM daily on days 1, 4 and 7 (n = 112). Each patient was scanned first using THYROGEN, then scanned using thyroid hormone withdrawal. The group receiving the THYROGEN 0.9 mg IM x 2 regimen was 63% female/27% male, had a mean age 44 years, and generally had low-stage papillary or follicular cancer (AJCC/TNM Stage I 61%, Stage II 19%, Stage III 14%, Stage IV 5%). The group receiving the THYROGEN 0.9 mg IM x 3 regimen was 66% female/34% male, had a mean age 50 years, and generally had low-stage papillary or follicular cancer (AJCC/TNM Stage I 50%, Stage II 20%, Stage III 20%, Stage IV 9%). The amount of radioiodine used for scanning was 4 mCi ± 10%, and scanning times were lengthened in some patients to capture adequate images (30 minute scans, or 140,000 counts). Scan pairs were assessed by blinded readers. Study results are presented in Table 2.
Table 2: Concordance of Positive Thyroid Scans Following THYROGEN Treatment with Scans Following Thyroid Hormone Withdrawal
|
|
Number of Scan Pairs by Disease Category |
Concordance of scan pairs between THYROGEN scan and thyroid hormone withdrawal scan |
|
Study 1 (0.9 mg IM qd x2) |
|
|
|
Positive for remnant or cancer in thyroid bed |
48 |
81% |
|
Positive for metastatic disease |
15 |
73% |
|
Total positive
withdrawal scans |
63 |
79% |
|
|
||
|
Study 2 (0.9 mg IM qd x 2) |
|
|
|
Positive for remnant or cancer in thyroid bed |
35 |
86% |
|
Positive for metastatic Disease |
9 |
67% |
|
Total positive
withdrawal scans |
44 |
82% |
Across the two clinical studies, and scoring all false positives in favor of thyroid hormone withdrawal, the majority of positive scans using THYROGEN and thyroid hormone withdrawal were concordant. The THYROGEN scan failed to detect remnant and/or cancer localized to the thyroid bed in 17% (14/83) of patients in whom it was detected by a scan after thyroid hormone withdrawal. In addition, the THYROGEN scan failed to detect metastatic disease in 29% (7/24) of patients in whom it was detected by a scan after thyroid hormone withdrawal.
Thyroglobulin (Tg) Results
THYROGEN Tg Testing Alone and in Combination with Diagnostic Whole Body Scanning: Comparison with Results after Thyroid Hormone Withdrawal
In anti-Tg antibody negative patients with a thyroid remnant or cancer (as defined by a withdrawal Tg ≥ 2.5 ng/mL or a positive scan [after thyroid hormone withdrawal or after radioiodine therapy]), the THYROGEN Tg was positive (≥ 2.5 ng/mL) in 69% (40/58) of patients after 2 doses of THYROGEN.
In these same patients, adding the whole body scan increased the detection rate of thyroid remnant or cancer to 84% (49/58) of patients after 2 doses of THYROGEN.
Among patients with metastatic disease confirmed by a post-treatment scan or by lymph node biopsy (35 patients), THYROGEN Tg was positive (≥ 2.5 ng/mL) in all 35 patients, while Tg on thyroid hormone suppressive therapy was positive ( ≥ 2.5 ng/mL) in 79% of these patients.
As with thyroid hormone withdrawal, the intra-patient reproducibility of THYROGEN testing with regard to both Tg stimulation and radioiodine imaging has not been studied.
Hypothyroid Signs and Symptoms
THYROGEN administration was not associated with the signs and symptoms of hypothyroidism that accompanied thyroid hormone withdrawal as measured by the Billewicz scale. Statistically significant worsening in all signs and symptoms were observed during the hypothyroid phase (p<0.01) (Figure 1).
Figure 1: Hypothyroid Symptom Assessment Billewicz Scale Diagnostic Indication 0.9 mg THYROGEN® q 24 hours x 2 doses vs. Thyroid Hormone Withdrawal Phase

14.2 Clinical Trials of THYROGEN as an Adjunct to Radioiodine Therapy to Achieve Thyroid Remnant Ablation
A randomized prospective clinical trial compared the rates of thyroid remnant ablation achieved after preparation of patients with thyroid hormone withdrawal or THYROGEN. Patients (n = 63) with low-risk, well-differentiated thyroid cancer who underwent near-total thyroidectomy were made euthyroid after surgery by receiving thyroid hormone replacement and were subsequently randomized to a thyroid hormone withdrawal or THYROGEN. Patients in the THYROGEN group received THYROGEN 0.9 mg IM daily on 2 consecutive days and radioiodine 24 hours after the second dose of THYROGEN. Patients in the thyroid hormone withdrawal group had the thyroid replacement withheld until they became hypothyroid. Patients in both groups received 100 mCi 131I ± 10% with the intent to ablate any thyroid remnant tissue. The primary endpoint of the study, was the rate of successful ablation, and was assessed 8 months later by a THYROGEN-stimulated radioiodine scan. Patients were considered successfully ablated if there was no visible thyroid bed uptake on the scan, or if visible, if uptake was less than 0.1%. Table 3 summarizes the results of this evaluation.
Table 3: Remnant Ablation in Clinical Trial of Patients with Well-Differentiated Thyroid Cancer
|
Group |
Mean Age (Yr) |
Gender (F:M) |
Cancer Type (Pap:Fol) |
Ablation Criterion (Measure at 8 Months) |
|
|
|
|
|
|
Thyroid Bed Activity <0.1% |
No Visible
Thyroid Bed Activity |
|
Thyroid Hormone Withdrawal |
43 |
24:6 |
29:1 |
28/28 (100%) |
24/28 (86%) |
|
THYROGEN |
44 |
26:7 |
30:3 |
32/32 (100%) |
24/32 (75%) |
The mean radiation dose to blood was 0.266±0.061 mGy/MBq in the THYROGEN group and 0.395±0.135 mGy/MBq in the thyroid hormone withdrawal group. Radioiodine residence time in remnant tissue was 0.9±1.3 hours in the THYROGEN group and 1.4±1.5 hours in the thyroid hormone withdrawal group. It is not known whether this difference in radiation exposure would convey a clinical benefit.
Patients who completed were followed up for a median duration of 3.7 years (range 3.4 to 4.4 years) following radioiodine ablation. Tg testing was also performed.The main objective of the follow-up study was to evaluate the status of thyroid remnant ablation by using THYROGEN-stimulated neck imaging. Of the fifty-one patients enrolled, forty eight patients received THYROGEN for remnant neck/whole body imaging and/or thyroglobulin testing. Only 43 patients had imaging. Patients were still considered to be successfully ablated if there was no visible thyroid bed uptake on the scan, or if visible, uptake was less than 0.1%. All patients from both original treatment groups who had scanning were found to still be ablated . Of 37 patients who were Tg–antibody negative, 16/17 (94%) of patients in the former thyroid hormone withdrawal group and 19/20 (95%) of patients in the former THYROGEN group maintained successful ablation measured as stimulated serum Tg levels of <2 ng/mL.
No patient had a definitive cancer recurrence during the 3.7 years of follow-up. Overall, 48/51 patients (94%) had no evidence of cancer recurrence, 1 patient had possible cancer recurrence (although it was not clear whether this patient had a true recurrence or persistent tumor from the regional disease noted at the start of the initial study), and 2 patients could not be assessed.
Two large prospective multi-center randomized studies compared THYROGEN to thyroid hormone withdrawal using two different doses of radioactive iodine in patients with differentiated thyroid cancer who had been thyroidectomized. In both studies, patients were randomized to 1 of 4 treatment groups: THYROGEN + 30 mCi 131I, THYROGEN + 100 mCi 131I, thyroid hormone withdrawal + 30 mCi 131I, or thyroid hormone withdrawal + 100 mCi 131I. Patients were assessed for efficacy (ablation success rates) at approximately 8 months.
The first study (Study A) randomized 438 patients (tumor stages T1-T3, Nx, N0 and N1, M0). Ablation success was defined as radioiodine uptake of <0.1% in the thyroid bed and stimulated thyroglobulin levels of < 2.0 ng/mL.
The second study (Study B) randomized 752 patients with low-risk thyroid cancer (tumor stages pT1 < 1 cm and N1 or Nx, pT1 >1-2 cm and any N stage, or pT2 N0, all patients M0). Ablation success was defined by neck ultrasound and stimulated thyroglobulin of ≤ 1.0 ng/mL.
Results for both trials are summarized below.
Table 4: Successful Remnant Ablation Rates in Study A
|
95% CI of difference in ablation rate (low-dose minus high dose): -10.2% to 2.6% |
|||
|
95% CI of difference in ablation rate (THYROGEN - Thyroid Hormone Withdrawal): -6.0% to 6.8% |
|||
|
THYROGEN |
Thyroid Hormone Withdrawal |
Total |
|
|
Low-dose radioiodine |
91/108 (84.3%) |
91/106 (85.8%) |
182/214 (85.0%) |
|
High-dose Radioiodine |
92/102 (90.2%) |
92/105 (87.6%) |
184/207 (88.9%) |
|
Total |
183/210 (87.1%) |
183/211 (86.7%) |
366/421 (86.9%) |
Table 5: Successful Remnant Ablation Rates in Study B
| 95% CI of difference in ablation rate (low-dose minus high dose): -5.8% to 0.9% | |||
| 95% CI of difference in ablation rate (THYROGEN minus Thyroid Hormone Withdrawal): -4.5% to 2.2% | |||
|
|
THYROGEN |
Thyroid Hormone Withdrawal |
Total |
|
Low-dose radioiodine |
160/177 (90.4%) |
156/170 (91.8%) |
316/347 (91.1%) |
|
High-dose Radioiodine |
159/171 (93.0%) |
156/166 (94.0%) |
315/337 (93.5%) |
|
Total
|
319/348 (91.6%) |
312/336 (92.9%) |
631/684 (92.3%) |
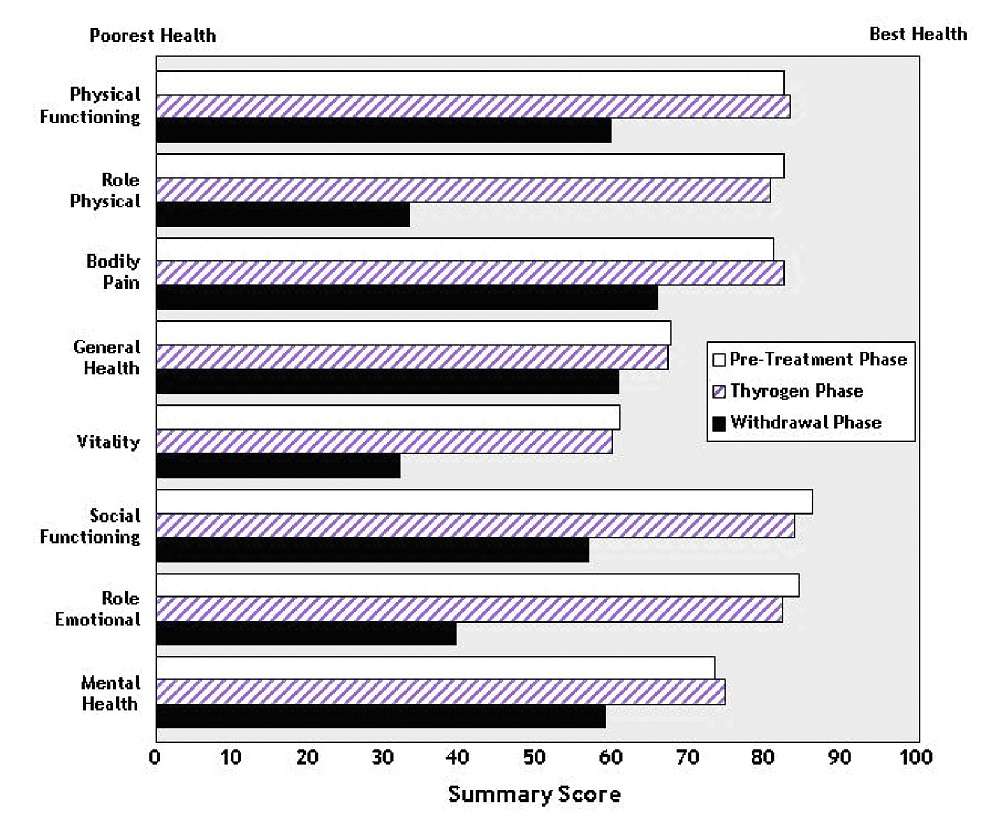
14.3 Quality of Life
Quality of Life (QOL) was measured during both the diagnostic study [ see Clinical Studies ( 14.1 )] and the ablation of thyroid remnant study [ see Clinical Studies ( 14.2 )] using the SF-36 Health Survey, a standardized, patient-administered instrument assessing QOL across eight domains measuring both physical and mental functioning. In the diagnostic study and in the remnant ablation study, following THYROGEN administration, little change from baseline was observed in any of the eight QOL domains of the SF-36. Following thyroid hormone withdrawal in the diagnostic study, statistically significant negative changes were noted in all eight QOL domains of the SF-36. The difference between treatment groups was statistically significant (p<0.0001) for all eight QOL domains, favoring THYROGEN over thyroid hormone withdrawal (Figure 2). In the remnant ablation study, following thyroid hormone withdrawal, statistically significant negative changes were noted in five of the eight QOL domains (physical functioning, role physical, vitality, social functioning and mental health).
Figure 2: SF-36
Health Survey Results
Quality of Life Domains
Diagnostic
Indication
16 HOW SUPPLIED/STORAGE AND HANDLING
THYROGEN (thyrotropin alfa for injection) is supplied as a sterile, non-pyrogenic, lyophilized product. It is available either in a two-vial kit or a four-vial kit. The two-vial kit contains two 1.1 mg vials of THYROGEN. The four-vial kit contains two 1.1 mg vials of THYROGEN, as well as two 10 mL vials of Sterile Water for Injection, USP.
NDC 58468-1849-4 (4-vial kit)
NDC 58468-0030-2 (2-vial kit)
THYROGEN is for intramuscular injection to the buttock. The lyophilized powder should be reconstituted immediately prior to use with 1.2 mL of Sterile Water for Injection, USP [see Dosage and Administration ( 2.2 )]. Each vial of THYROGEN and each vial of diluent, if provided, is intended for single use.
THYROGEN should be stored at 2-8ºC (36-46ºF)
If necessary, the reconstituted solution can be stored for up to 24 hours at a temperature between 2ºC and 8ºC, while avoiding microbial contamination.
Protect from light.
17 PATIENT COUNSELING INFORMATION
Adverse Reactions
- Inform patients that the most common adverse events from clinical experience were nausea and headache.
- Advise patients to seek immediate medical attention should they experience severe symptoms.
Important Information
- Prior to THYROGEN administration, counsel patients to seek care immediately for any neurologic symptoms occurring after administration of the drug.
- Inform patients for whom THYROGEN induced hyperthyroidism could have serious consequences, hospitalization for administration of THYROGEN and post-administrative observation should be considered.
Dosing and Administration
- Patients should be instructed that THYROGEN is for intramuscular administration into the buttock only. THYROGEN should not be administered intravenously.
- Inform patients the treatment regimen is two doses of THYROGEN administered at a 24 hour interval.
- Encourage patients to remain hydrated prior to treatment with THYROGEN.
Schedule of Procedures
- Inform patients that if diagnostic scanning will be performed, radioiodine will be given 24 hours after the second injection of THYROGEN, and patients should return for the scan 48 hours after radioiodine administration.
- Inform patients that if serum Tg testing is performed, blood will be drawn 72 hours or later after the second injection of THYROGEN.
- Inform patients that if remnant ablation is performed radioiodine will be administered 24 hours after the second injection of THYROGEN.
THYROGEN is manufactured and distributed by:
Genzyme Corporation
500 Kendall Street
Cambridge, MA 02142
(800) 745-4447
THYROGEN is a registered trademark of Genzyme Corporation.
Package Label - Principal Display Panel – 4 vial Carton
NDC 58468-1849-4
Thyrogen®
thyrotropin alfa for injection
0.9 mg/mL after reconstitution
For intramuscular injection only
Carton contains 2 vials of Thyrogen®
and 2 vials of diluent.
genzyme
Package Label - Principal Display Panel – 2 vial Carton
NDC 58468-0030-2
Thyrogen®
thyrotropin alfa for injection
0.9 mg/mL after reconstitution
For intramuscular injection only
Carton contains 2 vials of Thyrogen®
Dilute only with Sterile Water for Injection, USP.
genzyme
ThyrogenTHYROTROPIN ALFA INJECTION, POWDER, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ThyrogenTHYROTROPIN ALFA KIT
| ||||||||||||||||||||||||||||||||||||||||
