Tobramycin
X-GEN Pharmaceuticals, Inc.
X-GEN Pharmaceuticals, Inc.
Tobramycin for Injection USP, 1.2 gm†
FULL PRESCRIBING INFORMATION
PHARMACY BULK PACKAGE; NOT FOR DIRECT INFUSION
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Tobramycin for Injection USP and other antibacterial drugs, tobramycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Patients treated with tobramycin for injection USP and other aminoglycosides should be under close clinical observation, because these drugs have an inherent potential for causing ototoxicity and nephrotoxicity. Neurotoxicity, manifested as both auditory and vestibular ototoxicity, can occur. The auditory changes are irreversible, are usually bilateral, and may be partial or total. Eighth-nerve impairment and nephrotoxicity may develop, primarily in patients having preexisting renal damage and in those with normal renal function to whom aminoglycosides are administered for longer periods or in higher doses than those recommended. Other manifestations of neurotoxicity may include numbness, skin tingling, muscle twitching, and convulsions. The risk of aminoglycoside-induced hearing loss increases with the degree of exposure to either high peak or high trough serum concentrations. Patients who develop cochlear damage may not have symptoms during therapy to warn them of eighth-nerve toxicity, and partial or total irreversible bilateral deafness may continue to develop after the drug has been discontinued.
Rarely, nephrotoxicity may become apparent until the first few days after cessation of therapy. Aminoglycoside-induced nephrotoxicity usually is reversible.
Renal and eighth-nerve function should be closely monitored in patients with known or suspected renal impairment and also in those whose renal function is initially normal but who develop signs of renal dysfunction during therapy. Peak and trough serum concentrations of aminoglycosides should be monitored periodically during therapy to assure adequate levels and to avoid potentially toxic levels. Prolonged serum concentrations above 12 mcg/mL should be avoided. Rising trough levels (above 2 mcg/mL) may indicate tissue accumulation. Such accumulation, excessive peak concentrations, advanced age, and cumulative dose may contribute to ototoxicity and nephrotoxicity (see Precautions ). Urine should be examined for decreased specific gravity and increased excretion of protein, cells, and casts. Blood urea nitrogen, serum creatinine, and creatinine clearance should be measured periodically. When feasible, it is recommended that serial audiograms be obtained in patients old enough to be tested, particularly high-risk patients. Evidence of impairment of renal, vestibular, or auditory function requires discontinuation of the drug or dosage adjustment.
Tobramycin for injection USP should be used with caution in premature and neonatal infants because of their renal immaturity and the resulting prolongation of serum half-life of the drug.
Concurrent and sequential use of other neurotoxic and/or nephrotoxic antibiotics, particularly other aminoglycosides (eg. amikacin, streptomycin, neomycin, kanamycin, gentamicin, and paromomycin), cephaloridine, viomycin, polymyxin B, colistin, cisplatin, and vancomycin, should be avoided. Other factors that may increase patient risk are advanced age and dehydration.
Aminoglycosides should not be given concurrently with potent diuretics, such as ethacrynic acid and furosemide. Some diuretics themselves cause ototoxicity, and intravenously administered diuretics enhance aminoglycoside toxicity by altering antibiotic concentrations in serum and tissue.
Aminoglycosides can cause fetal harm when administered to a pregnant woman (see Precautions ).
Tobramycin sulfate, a water-soluble antibiotic of the aminoglycoside group, is derived from the actinomycete Streptomyces tenebrarius. Sterile tobramycin sulfate is supplied as a sterile dry powder and is intended for reconstitution with 30 mL of Sterile Water for Injection, USP. Sulfuric acid and/or sodium hydroxide may have been added during manufacture to adjust the pH. †Each vial contains tobramycin sulfate equivalent to 1200 mg of tobramycin. After reconstitution, the solution will contain 40 mg of tobramycin per mL. The product contains no preservative or sodium bisulfite.
Tobramycin sulfate is 0-3-amino-3-deoxy-a-D-glucopyranosyl-(1→4)-0-[2,6-diamino-2,3,6-trideoxy-a-D-ribo-hexopyranosyl-(1→6)]-2-deoxy-L-streptamine, sulfate (2:5)(salt) and has the chemical formula(C18H37N5O9)2•5H2SO4. The molecular weight is 1425.45. The structural formula for tobramycin is as follows:
The pharmacy bulk package of tobramycin for injection USP is a container of a sterile preparation for parenteral use that contains multiple single doses. It is intended for use in a pharmacy admixture program. Package use is restricted to the preparation of admixtures for intravenous infusion or to the filling of empty sterile syringes for intravenous injections for patients with individualized dosing requirements.
Tobramycin is rapidly absorbed following intramuscular administration. Peak serum concentrations of tobramycin occur between 30 and 90 minutes after intramuscular administration. Following an intramuscular dose of 1 mg/kg of body weight, maximum serum concentrations reach about 4 mcg/mL, and measurable levels persist for as long as 8 hours. Therapeutic serum levels are generally considered to range from 4 to 6 mcg/mL. When tobramycin is administered by intravenous infusion over a 1-hour period, the serum concentrations are similar to those obtained by intramuscular administration. Tobramycin is poorly absorbed from the gastrointestinal tract.
In patients with normal renal function, except neonates, tobramycin administered every 8 hours does not accumulate in the serum. However, in those patients with reduced renal function and in neonates, the serum concentration of the antibiotic is usually higher and can be measured for longer periods of time than in normal adults. Dosage for such patients must, therefore, be adjusted accordingly (see Dosage and Administration ).
Following parenteral administration, little, if any, metabolic transformation occurs, and tobramycin is eliminated almost exclusively by glomerular filtration. Renal clearance is similar to that of endogenous creatinine. Ultrafiltration studies demonstrate that practically no serum protein binding occurs. In patients with normal renal function, up to 84% of the dose is recoverable from urine in 8 hours and up to 93% in 24 hours.
Peak urine concentrations ranging from 75 to 100 mcg/mL have been observed following the intramuscular injection of a single dose of 1 mg/kg. After several days of treatment, the amount of tobramycin excreted in the urine approaches the daily dose administered. When renal function is impaired, excretion of tobramycin is slowed, and accumulation of the drug may cause toxic blood levels.
The serum half-life in normal individuals is 2 hours. An inverse relationship exists between serum half-life and creatinine clearance, and the dosage schedule should be adjusted according to the degree of renal impairment (see Dosage and Administration ). In patients undergoing dialysis, 25% to 70% of the administered dose may be removed, depending on the duration and type of dialysis.
Tobramycin can be detected in tissues and body fluids after parenteral administration. Concentrations in bile and stools ordinarily have been low, which suggests minimum biliary excretion. Tobramycin has appeared in low concentration in the cerebrospinal fluid following parenteral administration, and concentrations are dependent on dose, rate of penetration, and degree of meningeal inflammation. It has also been found in sputum, peritoneal fluid, synovial fluid, and abscess fluids, and it crosses the placental membranes. Concentrations in the renal cortex are several times higher than the usual serum levels.
Probenecid does not affect the renal tubular transport of tobramycin.
Microbiology–Tobramycin acts by inhibiting synthesis of protein in bacterial cells. In vitro tests demonstrate that tobramycin is bactericidal.
Tobramycin has been shown to be active against most strains of the following organisms both in vitro and in clinical infections as described in the Indications and Usage section:
| Aerobic Gram-positive microorganisms | ||
|---|---|---|
| Staphylococcus aureus | ||
| Aerobic Gram-negative microorganisms | |||||||
|---|---|---|---|---|---|---|---|
| Citrobacter | species | Enterobacter | species | Escherichia | coli | ||
| Klebsiella | species | Morganella | morganii | Pseudomonas | aeruginosa | ||
| Proteus | mirabilis | Proteus | vulgaris | Providencia | species | ||
| Serratis | species | ||||||
Aminoglycosides have a low order of activity against most gram-positive organisms, including Streptococcus pyogenes, Streptococcus pneumoniac, and enterococci.
Although most strains of enterococci demonstrate in vitro resistance, some strains in this group are susceptible. In vitro studies have shown that an aminoglycoside combined with an antibiotic that interferes with cell-wall synthesis affects some enterococcal strains synergistically. The combination of penicillin G and tobramycin results in a synergistic bactericidal effect in vitro against certain strains of Enterococcus faecalis. However, this combination is not synergistic against other closely related organisms, eg, Enterococcus faecium. Speciation of enterococci alone cannot be used to predict susceptibility. Susceptibility testing and tests for antibiotic synergisms are emphasized.
Cross resistance between aminoglycosides may occur.
Susceptibility Tests–
Diffusion techniques: Quantitative methods that require measurement of zone diameters give the most precise estimates of susceptibility of bacteria to antimicrobial agents. One such procedure is the National Committee for Clinical Laboratory Standards (NCCLS)-approved procedure.1 This method has been recommended for use with disks to test susceptibility to tobramycin. Interpretation involves correlation of the diameters obtained in the disk test with minimum inhibitory concentrations (MIC) for tobramycin.
Reports from the laboratory giving results of the standard single-disk susceptibility test with a 10-mcg tobramycin disk should be interpreted according to the following criteria:
| Zone | Diameter | (mm) | Interpretation | ||||
| >15 | (S) | Susceptible | |||||
| 13-14 | (I) | Intermediate | |||||
| <12 | (R) | Resistant | |||||
A report of “Susceptible” indicates that the pathogen is likely to be inhibited by generally achievable blood levels. A report of “Intermediate” suggests that the organism would be susceptible if high dosage is used or if the infection is confined to tissues and fluids in which high antimicrobial levels are obtained. A report of “Resistant” indicates that achievable concentrations are unlikely to be inhibitory and other therapy should be selected.
Standardized procedures require the use of laboratory control organisms. The 10-mcg tobramycin disk should give the following zone diameters:
| Organism | Zone | Diameter | (mm) | |||||
| E. | coli | ATCC | 25922 | 18-26 | ||||
| P. | aeruginosa | ATCC | 27853 | 19-25 | ||||
| S. | aureus | ATCC | 25923 | 19-29 | ||||
Dilution techniques: Broth and agar dilution methods, such as those recommended by the NCCLS,2 may be used to determine MICs of tobramycin. MIC test results should be interpreted according to the following criteria:
| MIC | (mcg/mL) | Interpretation | ||||
| < 4 | (S) | Susceptible | ||||
| 8 | (I) | Intermediate | ||||
| > 16 | (R) | Resistant | ||||
As with standard diffusion methods, dilution procedures require the use of laboratory control organisms. Tobramycin laboratory reagent should give the following MIC values:
| Organism | MIC | Range | (mcg/mL) | |||||
| E. | faecalis | ATCC | 29212 | 8-32 | ||||
| E. | coli | ATCC | 25922 | 0.25-1 | ||||
| P. | aeruginosa | ATCC | 27853 | 0.25-1 | ||||
| S. | aureus | ATCC | 29213 | 0.12-1 | ||||
Tobramycin for injection USP is indicated for the treatment of serious bacterial infections caused by susceptible strains of the designated microorganisms in the diseases listed below:
Septicemia in the pediatric patient and adult caused by P. aeruginosa, E. coli, and Klebsiella spp
Lower respiratory tract infections caused by P. aeruginosa, Klebsiella spp, Enterobacter spp, Serratia spp, E. coli, and S. aureus (penicillinase- and non-penicillinase-producing strains)
Serious central-nervous-system infections (meningitis) caused by susceptible organisms
Intra-abdominal infections, including peritonitis, caused by E. coli, Klebsiella spp, and Enterobacter spp
Skin, bone, and skin structure infections caused by P. aeruginosa, Proteus spp, E. coli, Klebsiella spp, Enterobacter spp, and S. aureus
Complicated and recurrent urinary tract infections caused by P. aeruginosa, Proteus spp (indole-positive and indole-negative), E. coli, Klebsiella spp, Enterobacter spp, Serratia spp, S. aureus, Providencia spp, and Citrobacter spp
Aminoglycosides, including tobramycin for injection USP, are not indicated in uncomplicated initial episodes of urinary tract infections unless the causative organisms are not susceptible to antibiotics having less potential toxicity. Tobramycin for injection USP may be considered in serious staphylococcal infections when penicillin or other potentially less toxic drugs are contraindicated and when bacterial susceptibility testing and clinical judgment indicate its use.
Bacterial cultures should be obtained prior to and during treatment to isolate and identify etiologic organisms and to test their susceptibility to tobramycin. If susceptibility tests show that the causative organisms are resistant to tobramycin, other appropriate therapy should be instituted. In patients in whom a serious life-threatening gram-negative infection is suspected, including those in whom concurrent therapy with a penicillin or cephalosporin and an aminoglycoside may be indicated, treatment with tobramycin for injection USP may be initiated before the results of susceptibility studies are obtained. The decision to continue therapy with tobramycin for injection USP should be based on the results of susceptibility studies, the severity of the infection, and the important additional concepts discussed in the Warnings box above.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of tobramycin and other antibacterial drugs, Tobramycin for Injection USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
A hypersensitivity to any aminoglycoside is a contraindication to the use of tobramycin. A history of hypersensitivity or serious toxic reactions to aminoglycosides may also contraindicate the use of any other aminoglycoside because of the known cross-sensitivity of patients to drugs in this class.
See Warnings box above.
Serious allergic reactions including anaphylaxis and dermatologic reactions including exfoliative dermatitis, toxic epidermal necrolysis, erythema multiforme, and Stevens-Johnson Syndrome have been reported rarely in patients on tobramycin therapy. Although rare, fatalities have been reported. (See CONTRAINDICATIONS ).
If an allergic reaction occurs, the drug should be discontinued and appropriate therapy instituted.
Prescribing tobramycin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Serum and urine specimens for examination should be collected during therapy, as recommended in the Warnings box. Serum calcium, magnesium, and sodium should be monitored.
Peak and trough serum levels should be measured periodically during therapy. Prolonged concentrations above 12 mcg/mL should be avoided. Rising trough levels (above 2 mcg/mL) may indicate tissue accumulation. Such accumulation, advanced age, and cumulative dosage may contribute to ototoxicity and nephrotoxicity. It is particularly important to monitor serum levels closely in patients with known renal impairment.
A useful guideline would be to perform serum level assays after 2 or 3 doses, so that the dosage could be adjusted if necessary, and at 3- to 4-day intervals during therapy. In the event of changing renal function, more frequent serum levels should be obtained and the dosage or the dosage interval adjusted according to the guidelines provided in the Dosage and Administration section.
In order to measure the peak level, a serum sample should be drawn about 30 minutes following intravenous infusion or 1 hour after an intramuscular injection. Trough levels are measured by obtaining serum samples at 8 hours or just prior to the next dose of tobramycin. These suggested time intervals are intended only as guidelines and may vary according to institutional practices. It is important, however, that there be consistency within the individual patient program unless computerized pharmacokinetic dosing programs are available in the institution. These serum-level assays may be especially useful for monitoring the treatment of severely ill patients with changing renal function or of those infected with less susceptible organisms or those receiving maximum dosage.
Neuromuscular blockade and respiratory paralysis have been reported in cats receiving very high doses of tobramycin (40 mg/kg). The possibility of prolonged or secondary apnea should be considered if tobramycin is administered to anesthetized patients who are also receiving neuromuscular blocking agents, such as succinyl choline, tubocurarine, or decamethonium, or to patients receiving massive transfusions of citrated blood. If neuromuscular blockade occurs, it may be reversed by the administration of calcium salts.
Cross-allergenicity among aminoglycosides has been demonstrated.
In patients with extensive burns or cystic fibrosis, altered pharmacokinetics may result in reduced serum concentrations of aminoglycosides. In such patients treated with tobramycin, measurement of serum concentration is especially important as a basis for determination of appropriate dosage.
Elderly patients may have reduced renal function that may not be evident in the results of routine screening tests, such as BUN or serum creatinine. A creatinine clearance determination may be more useful. Monitoring of renal function during treatment with aminoglycosides is particularly important in such patients.
An increased incidence of nephrotoxicity has been reported following concomitant administration of aminoglycoside antibiotics and cephalosporins.
Aminoglycosides should be used with caution in patients with muscular disorders, such as myasthenia gravis or parkinsonism, since these drugs may aggravate muscle weakness because of their potential curare-like effect on neuromuscular function.
Aminoglycosides may be absorbed in significant quantities from body surfaces after local irrigation or application and may cause neurotoxicity and nephrotoxicity.
Aminoglycosides have not been approved for intraocular and/or subconjunctival use. Physicians are advised that macular necrosis has been reported following administration of aminoglycosides, including tobramycin, by these routes.
See Warnings box regarding concurrent use of potent diuretics and concurrent and sequential use of other neurotoxic or nephrotoxic drugs.
The inactivation of tobramycin and other aminoglycosides by β-lactam-type antibiotics (penicillins or cephalosporins) has been demonstrated in vitro and in patients with severe renal impairment. Such inactivation has not been found in patients with normal renal function who have been given the drugs by separate routes of administration.
Therapy with tobramycin may result in overgrowth of nonsusceptible organisms. If overgrowth of nonsusceptible organisms occurs, appropriate therapy should be initiated.
See Indications and Usage and Dosage and Administration.
Patients should be counseled that antibacterial drugs including tobramycin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Tobramycin for Injection USP is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and not be treatable by tobramycin or any other antibacterial drugs in the future.
Pregnancy Category D – Aminoglycosides can cause fetal harm when administered to a pregnant woman. Aminoglycoside antibiotics cross the placenta, and there have been several reports of total irreversible bilateral congenital deafness in children whose mothers received streptomycin during pregnancy. Serious side effects to mother, fetus, or newborn have not been reported in the treatment of pregnant women with other aminoglycosides. If tobramycin is used during pregnancy or if the patient becomes pregnant while taking tobramycin, she should be apprised of the potential hazard to the fetus.
Diarrhea is a common problem caused by antibiotics, which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Neurotoxicity – Adverse effects on both the vestibular and auditory branches of the eighth nerve have been noted, especially in patients receiving high doses or prolonged therapy, in those given previous courses of therapy with an ototoxin, and in cases of dehydration. Symptoms include dizziness, vertigo, tinnitus, roaring in the ears, and hearing loss. Hearing loss is usually irreversible and is manifested initially by diminution of high-tone acuity. Tobramycin and gentamicin sulfates closely parallel each other in regard to ototoxic potential.
Nephrotoxicity – Renal function changes, as shown by rising BUN, NPN, and serum creatinine and by oliguria, cylindruria, and increased proteinuria, have been reported, especially in patients with a history of renal impairment who are treated for longer periods or with higher doses than those recommended. Adverse renal effects can occur in patients with initially normal renal function.
Clinical studies and studies in experimental animals have been conducted to compare the nephrotoxic potential of tobramycin and gentamicin. In some of the clinical studies and in the animal studies, tobramycin caused nephrotoxicity significantly less frequently than gentamicin. In some other clinical studies, no significant difference in the incidence of nephrotoxicity between tobramycin and gentamicin was found.
Other reported adverse reactions possibly related to tobramycin include anemia, granulocytopenia, and thrombocytopenia; and fever, rash, exfoliative dermatitis, itching, urticaria, nausea, vomiting, diarrhea, headache, lethargy, pain at the injection site, mental confusion, and disorientation. Laboratory abnormalities possibly related to tobramycin include increased serum transaminases (AST, ALT); increased serum LDH and bilirubin; decreased serum calcium, magnesium, sodium, and potassium; and leukopenia, leukocytosis, and eosinophilia.
To report SUSPECTED ADVERSE EVENTS, contact FDA at 1-800-FDA-1088 or www.fda.gov.
Signs and Symptoms - The severity of the signs and symptoms following a tobramycin overdose are dependent on the dose administered, the patient’s renal function, state of hydration, and age and whether or not other medications with similar toxicities are being administered concurrently. Toxicity may occur in patients treated more than 10 days, in adults given more than 5 mg/kg/day, in pediatric patients given more than 7.5 mg/kg/day, or in patients with reduced renal function where dose has not been appropriately adjusted.
Nephrotoxicity following the parenteral administration of an aminoglycoside is most closely related to the area under the curve of the serum concentration versus time graph. Nephrotoxicity is more likely if trough blood concentrations fail to fall below 2 mcg/mL and is also proportional to the average blood concentration. Patients who are elderly, have abnormal renal function, are receiving other nephrotoxic drugs, or are volume depleted are at greater risk for developing acute tubular necrosis. Auditory and vestibular toxicities have been associated with aminoglycoside overdose. These toxicities occur in patients treated longer than 10 days, in patients with abnormal renal function, in dehydrated patients, or in patients receiving medications with additive auditory toxicities. These patients may not have signs or symptoms or may experience dizziness, tinnitus, vertigo, and a loss of high-tone acuity as ototoxicity progresses. Ototoxicity signs and symptoms may not begin to occur until long after the drug has been discontinued.
Neuromuscular blockade or respiratory paralysis may occur following administration of aminoglycosides. Neuromuscular blockade, respiratory failure, and prolonged respiratory paralysis may occur more commonly in patients with myasthenia gravis or Parkinson’s disease. Prolonged respiratory paralysis may also occur in patients receiving decamethonium, tubocurarine, or succinyl choline. If neuromuscular blockade occurs, it may be reversed by the administration of calcium salts but mechanical assistance may be necessary.
If tobramycin were ingested, toxicity would be less likely because aminoglycosides are poorly absorbed from an intact gastrointestinal tract.
Treatment - In all cases of suspected overdosage, call your Regional Poison Control Center to obtain the most up-to-date information about the treatment of overdose. This recommendation is made because, in general, information regarding the treatment of overdose may change more rapidly than the package insert. In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
The initial intervention in a tobramycin overdose is to establish an airway and ensure oxygenation and ventilation. Resuscitative measures should be initiated promptly if respiratory paralysis occurs.
Patients who have received an overdose of tobramycin and who have normal renal function should be adequately hydrated to maintain a urine output of 3 to 5 mL/kg/hr. Fluid balance, creatinine clearance, and tobramycin plasma levels should be carefully monitored until the serum tobramycin level falls below 2 mcg/mL.
Patients in whom the elimination half-life is greater than 2 hours or whose renal function is abnormal may require more aggressive therapy. In such patients, hemodialysis may be beneficial.
This insert is for a Pharmacy Bulk Package and is intended for preparing intravenous admixtures only.
Tobramycin for Injection may be given intravenously. The patient’s pretreatment body weight should be obtained for calculation of correct dosage. It is desirable to measure both peak and trough serum concentrations (see Warnings box and Precautions ).
Administration for Patients With Normal Renal Function-Adults-With Serious Infections : 3 mg/kg/day in 3 equal doses every 8 hours (see Table 1 ).
Adults With Life-Threatening Infections : Up to 5 mg/kg/day may be administered in 3 or 4 equal doses (see Table 1 ). The dosage should be reduced to 3 mg/kg/day as soon as clinically indicated. To prevent increased toxicity due to excessive blood levels, dosage should not exceed 5 mg/kg/day unless serum levels are monitored (see Warnings box and Precautions ).
TABLE 1: DOSAGE SCHEDULE GUIDE FOR ADULTS WITH NORMAL RENAL FUNCTION (Dosage at 8-Hour Intervals)
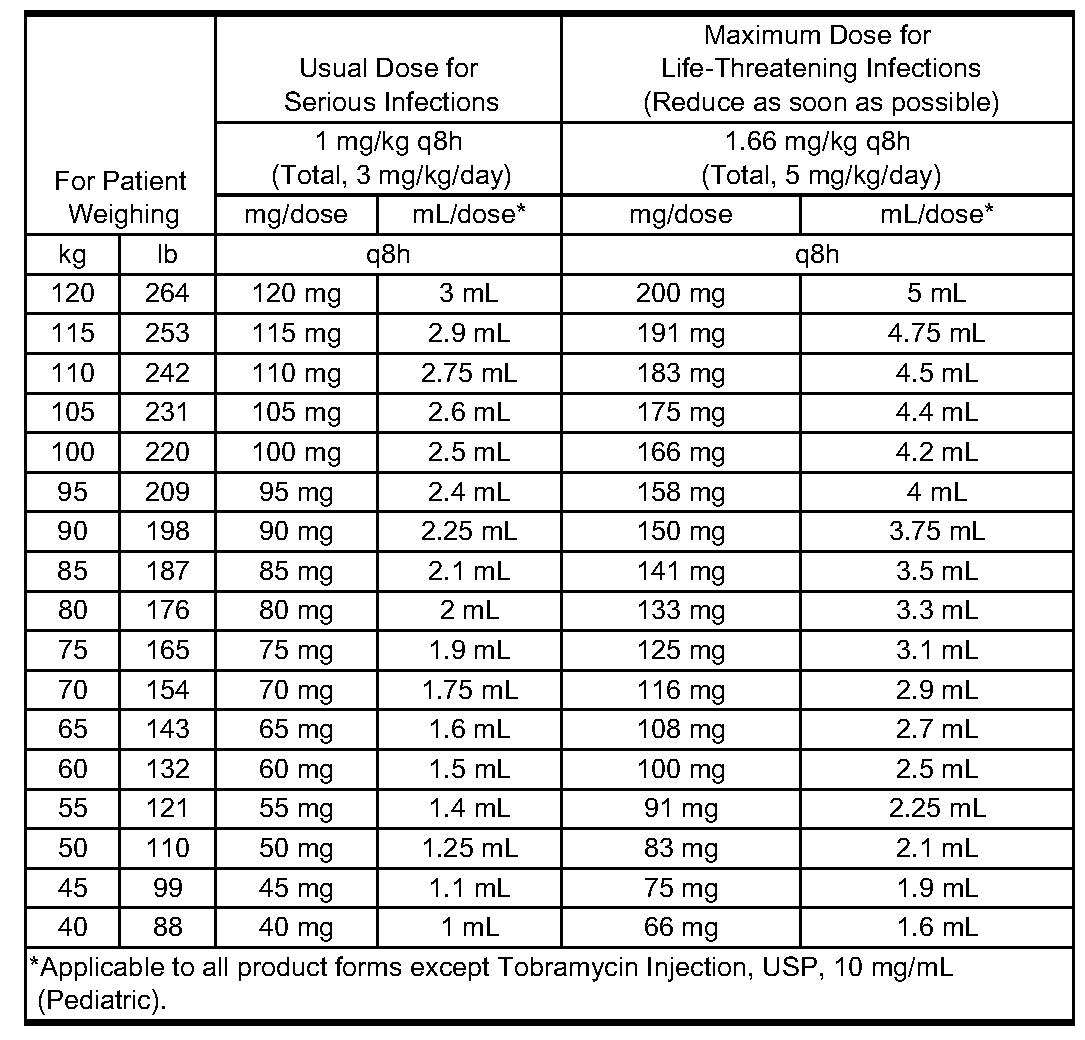
An alternate rough guide for determining reduced dosage at 8-hour intervals (for patients whose steady-state serum creatinine values are known) is to divide the normally recommended dose by the patient’s serum creatinine.
Normal dosage at prolonged intervals: If the creatinine clearance rate is not available and the patient’s condition is stable, a dosage frequency in hours for the dosage given in Table 1 can be determined by multiplying the patient’s serum creatinine by 6.
Dosage in Obese Patients - The appropriate dose may be calculated by using the patient’s estimated lean body weight plus 40% of the excess as the basic weight on which to figure mg/kg.
Intravenous Administration - For intravenous administration, the usual volume of diluent (0.9% Sodium Chloride Injection or 5% Dextrose Injection) is 50 to 100 mL for adult doses. For pediatric patients, the volume of diluent should be proportionately less than that for adults. The diluted solution usually should be infused over a period of 20 to 60 minutes. Infusion periods of less than 20 minutes are not recommended because peak serum levels may exceed 12 mcg/mL (see Warnings box ).
Tobramycin for injection USP should not be physically premixed with other drugs but should be administered separately according to the recommended dose and route.
Preparation and Storage –
Directions for Proper Use of Pharmacy Bulk Package – Not for direct infusion. The pharmacy bulk package is for use in the Hospital Pharmacy Admixture Service and only in a suitable work area, such as a laminar flow hood. Using aseptic technique, the closure may be penetrated only 1 time after reconstitution using a suitable sterile transfer device or dispensing set, which allows measured dispensing of the contents. Use of a syringe and needle is not recommended as it may cause leakage. After entry, entire contents of Pharmacy Bulk Package bottle should be dispensed within 24 hours.
Tobramycin for Injection is supplied as a dry powder. The contents of the Pharmacy Bulk Package bottle should be diluted with 30 mL of Sterile Water for Injection, USP, to provide a solution containing 40 mg of tobramycin per mL. Prior to reconstitution, the Pharmacy Bulk Package bottle should be stored at controlled room temperature, 20º to 25ºC (68º to 77ºF). After reconstitution, the solution should be kept in a refrigerator and used within 96 hours. If kept at room temperature, the solution must be used within 24 hours.
Prior to administration, parenteral drug products should be inspected visually for particulate matter and discoloration whenever solution and container permit.
Tobramycin for Injection, USP Pharmacy Bulk Package, containing tobramycin sulfate equivalent to 1.2 gm† of tobramycin (Dry Powder). (Pharmacy Bulk Package bottle NDC 39822-0409-1 and Six Pak NDC 39822-0409-6).
STORAGE
Store at controlled room temperature 20° to 25°C (68° to 77°F).[See USP Controlled Room Temperature].
1 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard--8th ed., CLSI document M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA. January, 2009.
2 Performance Standards for Antimicrobial Susceptibility Testing; 21st Informational Supplement. CLSI document M100-S21. Clinical and Laboratory Standards Institute, Wayne, PA. January, 2011.
3 Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard--10th ed., CLSI document M02-A10. Clinical and Laboratory Standards Institute, Wayne, PA. January, 2009.
†Each vial contains tobramycin sulfate equivalent to 1200 mg of tobramycin.
Manufactured for:
X-Gen Pharmaceuticals, Inc.
Big Flats, NY 14814
PREMIERProRx is a trademark of Premier, Inc., used under license.
Revised March 2013
TOBR-PR-PI-00
NDC 39822-0409-1
Tobramycin for Injection, USP
1.2 g Tobramycin Base Activity
For Intravenous Admixtures Only
Rx Only
1 .2 gram Vial
PremierPro RX

TobramycinTobramycin INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||