Topiramate
FULL PRESCRIBING INFORMATION: CONTENTS*
- TOPIRAMATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- INDICATIONS & USAGE
- TOPIRAMATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- TOPIRAMATE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
TOPIRAMATE DESCRIPTION
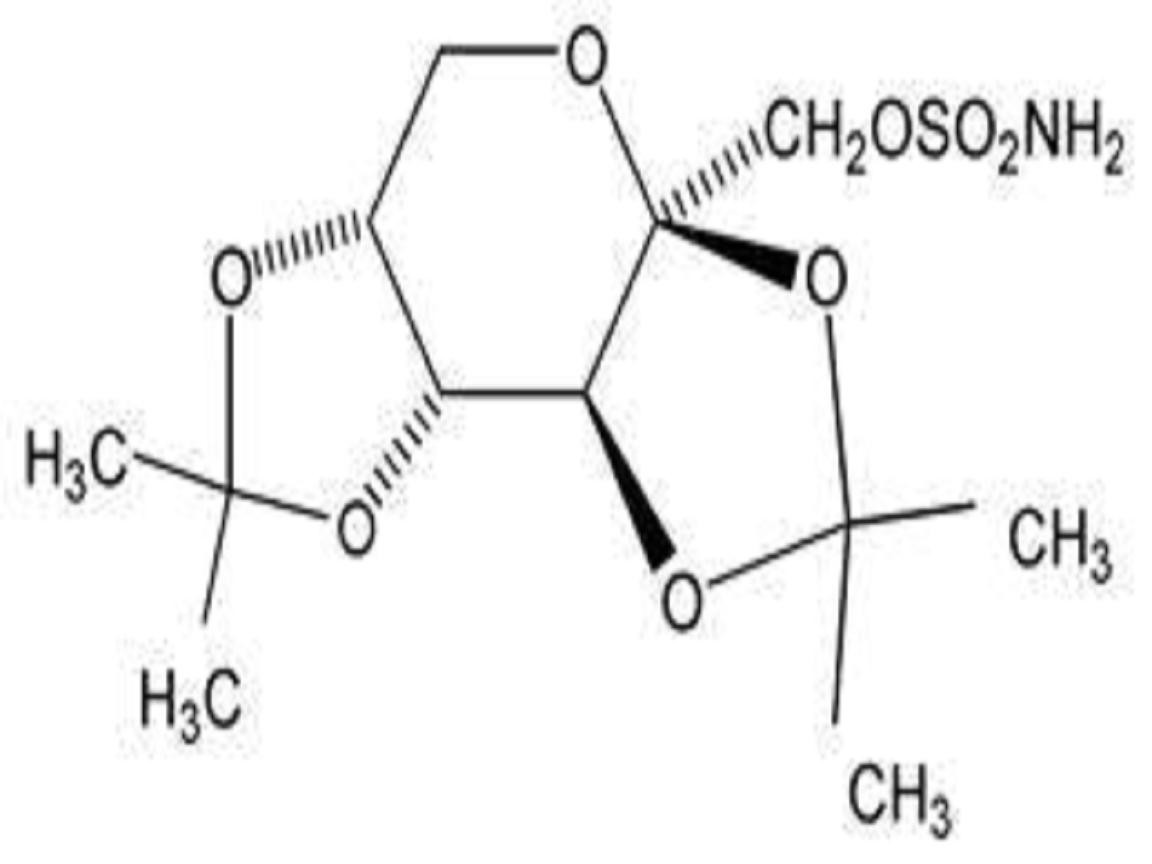
CLINICAL PHARMACOLOGY
Mechanism of Action:Pharmacodynamics:
Pharmacokinetics:
Metabolism and Excretion:
Pharmacokinetic Interactions (see also Drug Interactions):
PRECAUTIONS
Table 3
Special Populations:
Renal Impairment:
PRECAUTIONSAdjustment of Dose in Renal FailureDOSAGE AND ADMINISTRATION
Hemodialysis:
DOSAGE AND ADMINISTRATION
Hepatic Impairment:
Age, Gender, and Race:
Special Populations:Renal ImpairmentPRECAUTIONSAdjustment of Dose in Renal FailureDOSAGE AND ADMINISTRATION
Pediatric Pharmacokinetics:
CLINICAL STUDIES
Epilepsy
Monotherapy Controlled Trial
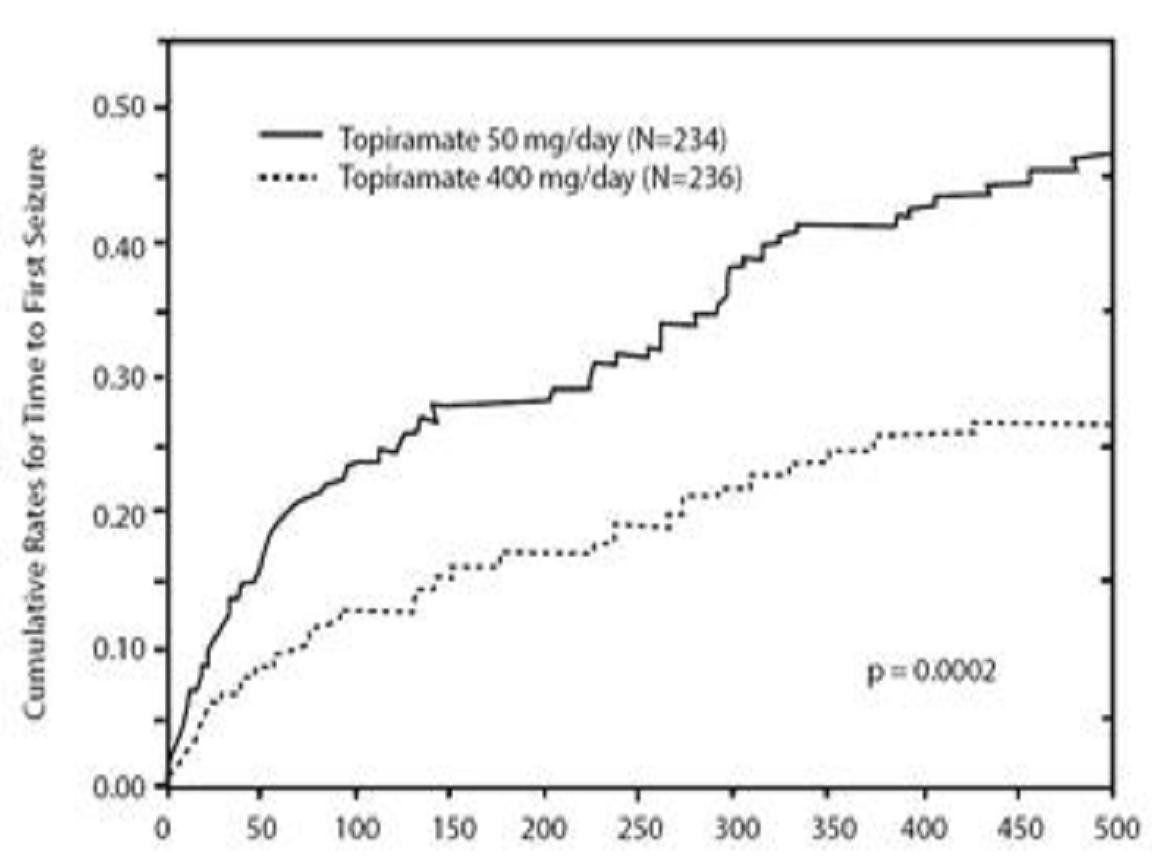
Adjunctive Therapy Controlled Trials in Adult Patients With Partial Onset Seizures
Adjunctive Therapy Controlled Trial in Pediatric Patients Ages 2 to 16 Years With Partial Onset Seizures
Adjunctive Therapy Controlled Trial in Patients With Primary Generalized Tonic-Clonic Seizures
Adjunctive Therapy Controlled Trial in Patients With Lennox-Gastaut Syndrome
INDICATIONS & USAGE
Monotherapy EpilepsyAdjunctive Therapy Epilepsy
TOPIRAMATE CONTRAINDICATIONS
WARNINGS
Metabolic AcidosisAcute Myopia and Secondary Angle Closure Glaucoma
Oligohidrosis and Hyperthermia
Withdrawal of AEDs
Cognitive/Neuropsychiatric Adverse Events
ADVERSE REACTIONSTable 4Table 6
Sudden Unexplained Death in Epilepsy (SUDEP)
PRECAUTIONS
DOSAGE AND ADMINISTRATION
INFORMATION FOR PATIENTS
PRECAUTIONS:Kidney Stones
LABORATORY TESTS
WARNINGSDRUG INTERACTIONS
Antiepileptic Drugs
Table 3
PRECAUTIONS
Other Drug Interactions
CNS Depressants:
Oral Contraceptives:
Hydrochlorothiazide (HCTZ):
Pioglitazone:
Lithium:
Haloperidol:
Amitriptyline:
Sumatriptan:
Risperidone:
Propranolol:
Dihydroergotamine:
Others:
Drug/Laboratory Test Interactions
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Pregnancy Category C.LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
WARNINGSGERIATRIC USE
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATIONRace and Gender Effects:
TOPIRAMATE ADVERSE REACTIONS
Monotherapy Epilepsy
Table 4
Table 5
Adjunctive Therapy Epilepsy
Table 6Table 8
Table 9
Incidence in Epilepsy Controlled Clinical Trials Adjunctive TherapyPartial Onset Seizures, Primary Generalized Tonic-Clonic Seizures, and Lennox-Gastaut Syndrome
Other Adverse Events Observed During Double-Blind Adjunctive Therapy Epilepsy Trials
Incidence in Study 119Add-On TherapyAdults with Partial Onset Seizures
Table 7
Other Adverse Events Observed During All Epilepsy Clinical Trials
Autonomic Nervous System Disorders:
Body as a Whole:
Cardiovascular Disorders, General:Infrequent:
Central & Peripheral Nervous System Disorders:Infrequent:
Gastrointestinal System Disorders:
Heart Rate and Rhythm Disorders:
Liver and Biliary System Disorders:
Metabolic and Nutritional Disorders:Infrequent
Musculoskeletal System Disorders:
Neoplasms:
Platelet, Bleeding, and Clotting Disorders:
Psychiatric Disorders:
Red Blood Cell Disorders:
Reproductive Disorders, Male:
Skin and Appendages Disorders
Special Senses Other, Disorders:
Urinary System Disorders:Infrequent:
Vascular (Extracardiac) Disorders:
Vision Disorders:
Rare:
White Cell and Reticuloendothelial System Disorders:
Postmarketing and Other Experience
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
WARNINGS
DOSAGE & ADMINISTRATION
EpilepsyMonotherapy Use
Adjunctive Therapy Use
Adults (17 Years of Age and Over) - Partial Seizures, Primary Generalized Tonic-Clonic Seizures, or Lennox-Gastaut Syndrome
CLINICAL STUDIESAdjunctive Therapy Controlled Trials in Patients With Primary Generalized Tonic-Clonic Seizures
Pediatric Patients (Ages 2 - 16 Years) - Partial Seizures, Primary Generalized Tonic-Clonic Seizures, or Lennox-Gastaut Syndrome
CLINICAL STUDIESAdjunctive Therapy Controlled Trial in Patients With Primary Generalized Tonic-Clonic Seizures
Patients with Renal Impairment:
Geriatric Patients (Ages 65 Years and Over):
DOSAGE AND ADMINISTRATION:Patients with Renal ImpairmentCLINICAL PHARMACOLOGY:Special Populations:Age, Gender, and Race
Patients Undergoing Hemodialysis:
Patients with Hepatic Disease:
HOW SUPPLIED
STORAGE AND HANDLING
INFORMATION FOR PATIENTS
What do TOPIRAMATE Tablets look like?
TOPIRAMATE Tablets
Note: The pictures above show the shapes and lettering of TOPIRAMATE tablets. The wording describes the strength and colors of the medication. Before taking your medicine, it is important to compare the tablets you receive from your healthcare professional or pharmacist with these pictures to make sure you have received the correct medicine.
What is TOPIRAMATE?
-
● alone to treat seizures in patients 10 years and older
-
● with other medicines to treat seizures in adults and children over age 2
Do not take TOPIRAMATE if you are allergic to anything in it.
What Should I Tell My Healthcare Professional Before Taking TOPIRAMATE?
Tell your healthcare professional about all of your medical conditions, including if you
-
● have kidney problems, especially kidney stones, or are getting kidney dialysis
-
● have a history of metabolic acidosis (blood and body fluid abnormality)
-
● have liver problems
-
● have osteoporosis (weak or brittle bones) and/or soft bones (osteomalacia) or decreased bone density (osteopenia)
-
● have lung or breathing problems
-
● have eye problems, especially glaucoma
-
● have diarrhea
-
● have a growth problem
-
● are on a diet high in fat called a ketogenic diet
-
● are having surgery
-
● are pregnant or planning to become pregnant. It is not known if TOPIRAMATE can harm your unborn baby.
-
● are breastfeeding. TOPIRAMATE may pass into your milk. Talk to your healthcare professional about the best way to feed your baby while taking TOPIRAMATE.
-
● suffer from depression, mood problems or suicidal thoughts or behavior
-
● other medicines that impair or decrease your thinking, concentration, or muscle coordination (e.g. central nervous system depressant medicines).
-
● birth control pills. TOPIRAMATE may make your birth control pills less effective. Tell your healthcare professional if your menstrual bleeding changes while you are taking birth control pills and TOPIRAMATE.
How Should I Take TOPIRAMATE?
-
● Take TOPIRAMATE exactly as prescribed. Your healthcare professional will usually start you on a low dose of TOPIRAMATE and slowly increase your dose until the best dose is found for you.
-
● TOPIRAMATE Tablets should be swallowed whole. Avoid, chewing the tablets as they may leave a bitter taste.
-
● Never store any medicine and food mixture for use at a later time.
-
● TOPIRAMATE can be taken before, during, or after a meal. Drink plenty of fluids during the day to prevent kidney stones while taking TOPIRAMATE.
-
● If you take too much TOPIRAMATE, call your healthcare professional or poison control center right away or go to an emergency room.
-
● If you miss a single dose of TOPIRAMATE, take it as soon as you can. However, if you are within 6 hours of taking your next scheduled dose, wait until then to take your usual dose of TOPIRAMATE, and skip the missed dose. Do not double your dose. If you have missed more than one dose, you should call your healthcare professional for advice.
-
● Do not stop taking TOPIRAMATE unless a healthcare professional tells you to stop taking TOPIRAMATE. Your healthcare professional will tell you how to slowly stop taking TOPIRAMATE.
-
● If you are taking Topiramate or other antiepileptic drugs for epilepsy or seizures, you may need to avoid activities where loss of consciousness (passing out) could result in serious danger to yourself or those around you (including swimming, driving a car, climbing in high places, etc.).Talk to your doctor before engaging in such activities.
-
● Unless prescribed by your healthcare professional, you should avoid other medicines that also impair or decrease your thinking, concentration, or muscle coordination (e.g. central nervous system depressant medicines).
-
● You should avoid drinking alcohol while taking TOPIRAMATE. Alcohol with TOPIRAMATE can make side effects such as sleepiness and dizziness worse.
-
● Do not drive a car or operate heavy machinery until you know how TOPIRAMATE affects you. TOPIRAMATE can impair your thinking, motor skills, and/or vision.
TOPIRAMATE may cause the following side effects which can be serious:
-
● metabolic acidosis.Metabolic acidosis is a condition that happens when there is too much acid in your blood. Metabolic acidosis can cause symptoms such as tiredness, loss of appetite, irregular heartbeat, and impaired consciousness.Call your healthcare professional right away if you get these symptoms with TOPIRAMATE.Your healthcare professional should do a blood test (measurement of serum bicarbonate) to monitor your bicarbonate level while you are taking TOPIRAMATE.
-
● eye problems. Serious eye problems include:
-
● a sudden decrease in vision (acute myopia) with or without eye pain and
-
● a blockage of fluid in the eye causing increased pressure in the eye (secondary angle closure glaucoma).
-
● decreased sweating (oligohidrosis) and increased body temperature (fever).
-
● effects on thinking and alertness.TOPIRAMATE may affect thinking skills and cause confusion, problems with concentration, attention, memory, and/or speech.
-
● dizziness or loss of muscle coordinationin patients who take TOPIRAMATE alone or with other seizure medicines.
-
● high blood ammonia levels and effects on mental activities.High ammonia in the blood can affect your mental activities and decrease alertness, can make you feel tired or fatigued, or can cause vomiting. This has happened when TOPIRAMATE has been used with a medicine called valproic acid.
-
● kidney stones.Drink plenty of fluids when taking TOPIRAMATE to decrease your chances of getting kidney stones.
-
● tingling of the arms and legs (paresthesia)is a common side effect of TOPIRAMATE.
What Should I Do If I Get Pregnant While Taking TOPIRAMATE?
How Should I Store TOPIRAMATE?
-
● Store at 20to 25(68to 77
-
● Keep TOPIRAMATE and all medicines out of the reach of children.
What Are the Ingredients of TOPIRAMATE?
Active Ingredient:
Inactive Ingredients:
-
● Tablets - lactose monohydrate, microcrystalline cellulose, pre-gelatinized starch, lactose monohydrate, sodium starch glycolate, magnesium stearate, opadry white (titanium dioxide, hypromellose 3cp, hypromellose 6cp, PEG 400, polysorbate 80) for 25 mg tablets, opadry yellow (titanium dioxide, hypromellose 3cp, hypromellose 6cp, PEG 400, polysorbate 80, iron oxide yellow) for 50 mg tablets, opadry yellow , hypromellose 6cp titanium dioxide, PEG 400, iron oxide yellow, polysorbate 80, iron oxide red) for 100 mg tablets and), opadry pink (titanium dioxide, hypromellose 6cp, PEG 400, iron oxide red) for 200 mg tablets.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

TopiramateTopiramate TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!