Topiramate
FULL PRESCRIBING INFORMATION: CONTENTS*
- TOPIRAMATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- INDICATIONS & USAGE
- TOPIRAMATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- TOPIRAMATE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
TOPIRAMATE DESCRIPTION
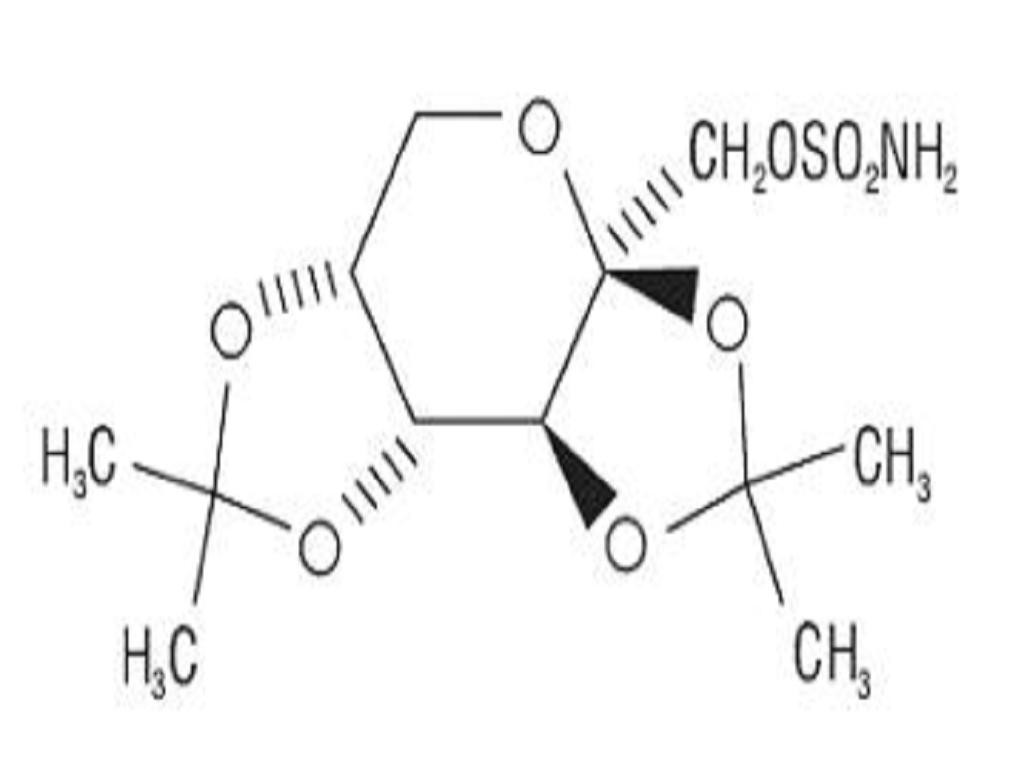
CLINICAL PHARMACOLOGY
Mechanism of Action:
Pharmacodynamics:
Pharmacokinetics:
Metabolism and Excretion:
Pharmacokinetic Interactions
PRECAUTIONS (Table 4)
Special Populations:
Renal Impairment:
PRECAUTIONS: Adjustment of Dose in Renal FailureDOSAGE AND ADMINISTRATION
Hemodialysis:
DOSAGE AND ADMINISTRATION
Hepatic Impairment:
Age, Gender, and Race:
Special Populations: Renal ImpairmentPRECAUTIONS: Adjustment of Dose in Renal FailureDOSAGE AND ADMINISTRATION
Pediatric Pharmacokinetics:
CLINICAL STUDIES
Epilepsy
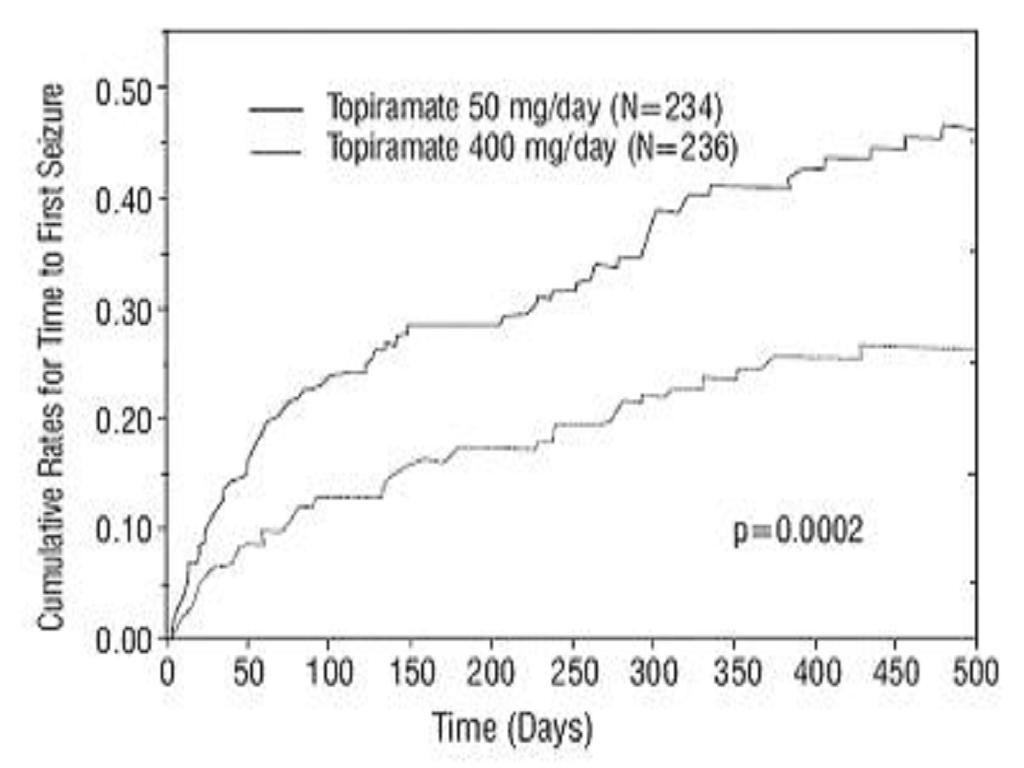
Monotherapy Controlled Trial
Figure 1
Adjunctive Therapy Controlled Trials in Patients With Partial Onset Seizures
Table 1
Adjunctive Therapy Controlled Trial in Pediatric Patients Ages 2 to 16 Years With Partial Onset Seizures
Adjunctive Therapy Controlled Trial in Patients With Primary Generalized Tonic-Clonic Seizures
Adjunctive Therapy Controlled Trial in Patients With Lennox-Gastaut Syndrome
Table 2
INDICATIONS & USAGE
Monotherapy EpilepsyAdjunctive Therapy Epilepsy
TOPIRAMATE CONTRAINDICATIONS
WARNINGS
Acute Myopia and Secondary Angle Closure GlaucomaOligohidrosis and Hyperthermia
Suicidal Behavior and Ideation
IndicationPlacebo Patients with Events Per 1000 PatientsDrug Patients with Events Per 1000 PatientsRelative Risk: Incidence of Events in Drug Patients/Incidence in Placebo PatientsRisk Difference: Additional Drug Patients with Events Per 1000 Patients
Metabolic Acidosis
Cognitive/Neuropsychiatric Adverse Events
Adults
Cognitive-Related Dysfunction
ADVERSE REACTIONSTable 5Table 7
Psychiatric/Behavioral Disturbances
Somnolence/Fatigue
Pediatric Patients
Withdrawal of AEDs
Sudden Unexplained Death in Epilepsy (SUDEP)
PRECAUTIONS
Hyperammonemia and Encephalopathy Associated with Concomitant Valproic Acid UseKidney Stones
Paresthesia
Adjustment of Dose in Renal Failure
DOSAGE AND ADMINISTRATION
Decreased Hepatic Function
INFORMATION FOR PATIENTS
PRECAUTIONS: Kidney Stones
PRECAUTIONS: Pregnancy: Pregnancy Category C
LABORATORY TESTS
WARNINGSDRUG INTERACTIONS
Antiepileptic Drugs
Table 4
Table 4
PRECAUTIONS, Hyperammonemia and Encephalopathy Associated with Concomitant Valproic Acid Use
Other Drug Interactions
Digoxin
CNS Depressants
Oral Contraceptives
Hydrochlorothiazide (HCTZ)
Pioglitazone
Lithium
Haloperidol
Amitriptyline
Sumatriptan
Risperidone
Propranolol
Dihydroergotamine
Others
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Pregnancy Category C.LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
WARNINGSGERIATRIC USE
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATIONRace and Gender Effects:
TOPIRAMATE ADVERSE REACTIONS
Monotherapy Epilepsy
Table 5
Table 6
Adjunctive Therapy Epilepsy
Table 7Table 9
Table 10
Incidence in Epilepsy Controlled Clinical Trials - Adjunctive TherapyPartial Onset Seizures, Primary Generalized Tonic-Clonic Seizures, and Lennox-Gastaut Syndrome
Table 7 Table 10
Other Adverse Events Observed During Double-Blind Epilepsy Adjunctive Therapy Trials
Incidence in Study 119Add-On TherapyAdults with Partial Onset Seizures
Table 8
Other Adverse Events Observed During All Epilepsy Clinical Trials
Autonomic Nervous System Disorders:
Body as a Whole:
Cardiovascular Disorders, General:
Central & Peripheral Nervous System Disorders:
Gastrointestinal System Disorders:
Heart Rate and Rhythm Disorders:
Liver and Biliary System Disorders:
Metabolic and Nutritional Disorders:
Musculoskeletal System Disorders:
Neoplasms:
Platelet, Bleeding, and Clotting Disorders:
Psychiatric Disorders:
Red Blood Cell Disorders:
Reproductive Disorders, Male:
Skin and Appendages Disorders:
Special Senses Other, Disorders:
Urinary System Disorders:
Vascular (Extracardiac) Disorders:
Vision Disorders:
White Cell and Reticuloendothelial System Disorders:
Postmarketing and Other Experience
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
WARNINGS
DOSAGE & ADMINISTRATION
EpilepsyMonotherapy Use
Adjunctive Therapy Use
Adults (17 Years of Age and Over) - Partial Seizures, Primary Generalized Tonic-Clonic Seizures, or Lennox-Gastaut Syndrome
CLINICAL STUDIES, Adjunctive Therapy Controlled Trials in Patients With Primary Generalized Tonic-Clonic Seizures
Pediatric Patients (Ages 2 to 16 Years)Partial Seizures, Primary Generalized Tonic-Clonic Seizures, or Lennox-Gastaut Syndrome
CLINICAL STUDIES, Adjunctive Therapy Controlled Trials in Patients With Primary Generalized Tonic-Clonic Seizures
Patients with Renal Impairment:
Geriatric Patients (Ages 65 Years and Over):
DOSAGE AND ADMINISTRATION: Patients with Renal ImpairmentCLINICAL PHARMACOLOGY: Special Populations: Age, Gender, and Race
Patients Undergoing Hemodialysis:
Patients with Hepatic Disease:
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
Topiramate Tablets
What is the most important information I should know about topiramate tablets?
-
● Topiramate tablets may cause eye problems . Serious eye problems include:
-
● any sudden decrease in vision with or without eye pain and redness
-
● a blockage of fluid in the eye causing increased pressure in the eye (secondary angle closure glaucoma).
-
● These eye problems can lead to permanent loss of vision if not treated. You should call your healthcare provider right away if you have any new eye symptoms.
-
● Topiramate tablets may cause decreased sweating and increased body temperature (fever).People, especially children, should be watched for signs of decreased sweating and fever, especially in hot temperatures. Some people may need to be hospitalized for this condition.
-
● Like other antiepileptic drugs, topiramate tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
-
● Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
-
● thoughts about suicide or dying
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
-
● Stopping topiramate tablets suddenly can cause serious problems.
-
● Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
-
● Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
-
● Keep all follow-up visits with your healthcare provider as scheduled.
-
● Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
-
● to treat certain types of seizures (partial onset seizures and primary generalized tonic-clonic seizures) in people 10 years and older
-
● with other medicines to treat certain types of seizures (partial onset seizures, primary generalized tonic-clonic seizures, and seizures associated with Lennox-Gastaut syndrome) in adults and children 2 years and older
Before taking topiramate tablets, tell your healthcare provider about all your medical conditions, including if you:
-
● have or have had depression, mood problems or suicidal thoughts or behavior
-
● have kidney problems, kidney stones, or are getting kidney dialysis
-
● have a history of metabolic acidosis (too much acid in the blood)
-
● have liver problems
-
● have osteoporosis, soft bones, or decreased bone density
-
● have lung or breathing problems
-
● have eye problems, especially glaucoma
-
● have diarrhea
-
● have a growth problem
-
● are on a diet high in fat and low in carbohydrates, which is called a ketogenic diet
-
● are having surgery
-
● are pregnant or plan to become pregnant. It is not known if topiramate tablets will harm your unborn baby. If you become pregnant while taking topiramate tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic medicine during pregnancy.
-
● are breastfeeding. It is not known if topiramate passes into breast milk and if it can harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take topiramate tablets.
-
● Valproic acid
-
● any medicines that impair or decrease your thinking, concentration, or muscle coordination.
-
● birth control pills. Topiramate tablets may make your birth control pills less effective. Tell your healthcare provider if your menstrual bleeding changes while you are taking birth control pills and topiramate tablets.
How should I take topiramate tablets?
-
● Take topiramate tablets exactly as prescribed.
-
● Your healthcare provider may change your dose. Do not change your dose without talking to your healthcare provider.
-
● Topiramate tablets should be swallowed whole. Do not chew the tablets. They may leave a bitter taste.
-
● Do not store any medicine and food mixture for later use.
-
● Topiramate tablets can be taken before, during, or after a meal. Drink plenty of fluids during the day. This may help prevent kidney stones while taking topiramate tablets.
-
● If you take too many topiramate tablets, call your healthcare provider or poison control center right away or go to the nearest emergency room.
-
● If you miss a single dose of topiramate tablets, take it as soon as you can. However, if you are within 6 hours of taking your next scheduled dose, wait until then to take your usual dose of topiramate tablets, and skip the missed dose. Do not double your dose. If you have missed more than one dose, you should call your healthcare professional for advice.
-
● Do not stop taking topiramate tablets without talking to your healthcare provider. Stopping topiramate tablets suddenly may cause serious problems. If you have epilepsy and you stop taking topiramate tablets suddenly, you may have seizures that do not stop. Your healthcare provider will tell you how to stop taking topiramate tablets slowly.
-
● Your healthcare provider may do blood tests while you take topiramate tablets.
-
● Do not drink alcohol while taking topiramate tablets. Topiramate tablets and alcohol can affect each other causing side effects such as sleepiness and dizziness.
-
● Do not drive a car or operate heavy machinery until you know how topiramate tablets affect you. Topiramate tablets can slow your thinking and motor skills.
-
● Metabolic Acidosis.Metabolic acidosis can cause:
-
● tiredness
-
● loss of appetite
-
● irregular heartbeat
-
● impaired consciousness
-
● High blood ammonia levels.High ammonia in the blood can affect your mental activities, slow your alertness, make you feel tired, or cause vomiting. This has happened when topiramate tablets are taken with a medicine called valproic acid.
-
● Kidney stones.Drink plenty of fluids when taking topiramate tablets to decrease your chances of getting kidney stones.
-
● Effects on Thinking and Alertness.Topiramate tablets may affect how you think, and cause confusion, problems with concentration, attention, memory, or speech. Topiramate tablets may cause depression or mood problems, tiredness, and sleepiness.
-
● Dizziness or Loss of Muscle Coordination.
-
● Call your healthcare provider right away if you have any of the symptoms above.
-
● tingling of the arms and legs (paresthesia)
-
● not feeling hungry
-
● nausea
-
● a change in the way foods taste
-
● diarrhea
-
● weight loss
-
● nervousness
-
● upper respiratory tract infection
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store topiramate tablets?
-
● Store topiramate tablets at 20to 25(68to 77to 30(59to 86
-
● Keep topiramate tablets in a tightly closed container
-
● Keep topiramate tablets dry and away from moisture
-
● Keep topiramate tablets and all medicines out of the reach of children.
What are the ingredients in topiramate tablets?
Active ingredient:
Inactive ingredients:
This Medication Guide has been approved by the U.S. Food and Drug Administration.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


TopiramateTopiramate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!