TOPIRAMATE
St Marys Medical Park Pharmacy
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use topiramate tablets safely and effectively. See full prescribing information for topiramate tablets Topiramate Tablets Rx Only Initial U.S. Approval - 1996RECENT MAJOR CHANGESRECENT MAJOR CHANGES - Warnings and Precautions (5.3) [04/2009] - Warnings and Precautions (5.8) [12/2009] INDICATIONS AND USAGEINDICATIONS AND USAGE Topiramate is an antiepileptic (AED) agent indicated for: - Monotherapy epilepsy: Initial monotherapy in patients greater than or equal to 10 years of age with partial onset or primary generalized tonic-clonic seizures (1.1). - Adjunctive therapy epilepsy: Adjunctive therapy for adults and pediatric patients (2 to 16 years of age) with partial onset seizures or primary generalized tonic-clonic seizures, and in patients greater than or equal to 2 years of age with seizures associated with Lennox-Gastaut syndrome (LGS) (1.2). DOSAGE AND ADMINISTRATIONDOSAGE AND ADMINISTRATION See DOSAGE AND ADMINISTRATION, Epilepsy: Adjunctive Therapy Use for additional details (2.1). Initial Dose Titration Recommended Dose Epilepsy monotherapy: adults and pediatric patients greater than or equal to 10 years (2.1) 50 mg/day in two divided doses The dosage should be increased weekly by increments of 50 mg for the first 4 weeks then 100 mg for weeks 5 to 6. 400 mg/day in two divided doses Epilepsy adjunctive therapy: adults with partial onset seizures or LGS (2.1) 25 to 50 mg/day The dosage should be increased weekly to an effective dose by increments of 25 to 50 mg. 200-400 mg/day in two divided doses Epilepsy adjunctive therapy: adults with primary generalized tonic-clonic seizures (2.1) 25 to 50 mg/day The dosage should be increased weekly to an effective dose by increments of 25 to 50 mg. 400 mg/day in two divided doses Epilepsy adjunctive therapy: pediatric patients with partial onset seizures, primary generalized tonic-clonic seizures or LGS (2.1) 25 mg/day (or less, based on a range of 1 to 3 mg/kg/day) nightly for the first week The dosage should be increased at 1- or 2-week intervals by increments of 1 to 3 mg/kg/day (administered in two divided doses). Dose titration should be guided by clinical outcome. 5 to 9 mg/kg/day in two divided doses DOSAGE FORMS AND STRENGTHSDOSAGE FORMS AND STRENGTHS Tablets: 25 mg, 50 mg, 100 mg, and 200 mg (3) CONTRAINDICATIONSCONTRAINDICATIONS None. WARNINGS AND PRECAUTIONSWARNINGS AND PRECAUTIONS - Acute myopia and secondary angle closure glaucoma: Untreated elevated intraocular pressure can lead to permanent visual loss. The primary treatment to reverse symptoms is discontinuation of topiramate as rapidly as possible (5.1). - Oligohidrosis and hyperthermia: Monitor decreased sweating and increased body temperature, especially in pediatric patients (5.2). - Suicidal behavior and ideation: Antiepileptic drugs increase the risk of suicidal behavior or ideation (5.3). - Metabolic acidosis: Baseline and periodic measurement of serum bicarbonate is recommended. Consider dose reduction or discontinuation of topiramate if clinically appropriate (5.4). - Cognitive/neuropsychiatric: Topiramate may cause cognitive dysfunction. Patients should use caution when operating machinery including automobiles. Depression and mood problems may occur in epilepsy and other populations (5.5). - Withdrawal of AEDs: Withdrawal of topiramate should be done gradually (5.6). - Hyperammonemia and encephalopathy associated with or without concomitant valproic acid use: Patients with inborn errors of metabolism or reduced mitochondrial activity may have an increased risk of hyperammonemia. Measure ammonia if encephalopathic symptoms occur (5.8). - Kidney stones: Use with other carbonic anhydrase inhibitors, other drugs causing metabolic acidosis, or in patients on a ketogenic diet should be avoided (5.9). Side EffectsADVERSE REACTIONS The most common (greater than or equal to 5% more frequent than placebo or low dose topiramate in monotherapy) adverse reactions in controlled, epilepsy clinical trials were paresthesia, anorexia, weight decrease, fatigue, dizziness, somnolence, nervousness, psychomotor slowing, difficulty with memory, difficulty with concentration/attention, and confusion.TO REPORT SUSPECTED ADVERSE REACTIONS, CONTACT TORRENT PHARMA INC. AT 1-269-544-2299 OR FDA AT 1-800-FDA-1088 OR WWW.FDA.GOV/MEDWATCH. DRUG INTERACTIONSDRUG INTERACTIONS Summary of antiepileptic drug (AED) interactions with topiramate (7.1). AED Co-administered AED Concentration Topiramate Concentration Phenytoin NC or 25% increasea 48% decrease Carbamazepine (CBZ) NC 40% decrease CBZ epoxideb NC NE Valproic acid 11% decrease 14% decrease Phenobarbital NC NE Primidone NC NE Lamotrigine NC at TPM doses up to 400 mg/day 13% decrease - Concomitant administration of valproic acid and topiramate has been associated with hyperammonemia with and without encephalopathy (5.7). - Oral contraceptives: Decreased contraceptive efficacy and increased breakthrough bleeding should be considered, especially at doses greater than 200 mg/day (7.3). - Metformin is contraindicated with metabolic acidosis, a possible effect of topiramate (7.4) - Lithium levels should be monitored when co-administered with high-dose topiramate (7.5) - Other Carbonic Anhydrase Inhibitors: monitor the patient for the appearance or worsening of metabolic acidosis (7.6)USE IN SPECIFIC POPULATIONSUSE IN SPECIFIC POPULATIONS - Renal Impairment: In renally impaired patients (creatinine clearance less than 70 mL/min/1.73 m2), one half of the adult dose is recommended (2.4). - Patients Undergoing Hemodialysis: Topiramate is cleared by hemodialysis. Dosage adjustment is necessary to avoid rapid drops in topiramate plasma concentration during hemodialysis (2.6). - Pregnancy: based on animal data, may cause fetal harm. To enroll in the North American Antiepileptic Drug Pregnancy Registry call 1-800-233-2334 (toll free) (8.1). - Geriatric Use: Dosage adjustment may be necessary for elderly with impaired renal function (8.5). See 17 for PATIENT COUNSELING INFORMATION and the FDA-approved Medication Guide Revised: 06/2010
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 TOPIRAMATE INDICATIONS AND USAGE 1.1 Monotherapy Epilepsy 1.2 Adjunctive Therapy Epilepsy
- 2. TOPIRAMATE DOSAGE AND ADMINISTRATION 2.1 Epilepsy 2.4 Patients with Renal Impairment 2.5 Geriatric Patients (Ages 65 Years and Over) 2.6 Patients Undergoing Hemodialysis 2.7 Patients with Hepatic Disease
- 3. DOSAGE FORMS AND STRENGTHS
- 4. TOPIRAMATE CONTRAINDICATIONS
- 5. WARNINGS AND PRECAUTIONS 5.1 Acute Myopia and Secondary Angle Closure Glaucoma 5.2 Oligohidrosis and Hyperthermia 5.3 Suicidal Behavior and Ideation 5.4 Metabolic Acidosis 5.5 Cognitive/Neuropsychiatric Side Effects 5.6 Withdrawal of Antiepileptic
- Table 2 Table 3 Table 4 Table 6 Table 7 6. TOPIRAMATE ADVERSE REACTIONS 6.1 Monotherapy Epilepsy 6.2 Adjunctive Therapy Epilepsy 6.3 Incidence in Epilepsy Controlled Clinical Trials – Adjunctive Therapy – Partial Onset Seizures, Primary Generalized Tonic-Clonic Seiz
- 7. DRUG INTERACTIONS 7.1 Antiepileptic Drugs 7.2 CNS Depressants 7.3 Oral Contraceptives 7.4 Metformin 7.5 Lithium 7.6 Other Carbonic Anhydrase Inhibitors
- 8. USE IN SPECIFIC POPULATIONS 8.1 Pregnancy
- 8.2 Labor and Delivery
- 8.3 Nursing Mothers
- 8.4 Pediatric Use
- 8.5 Geriatric Use 8.6 Race and Gender Effects 8.7 Renal Impairment 8.8 Patients Undergoing Hemodialysis
- 9. DRUG ABUSE AND DEPENDENCE 9.1 Controlled Substance
- 9.2 Abuse
- 9.3 Dependence
- 10 OVERDOSAGE
- 11. TOPIRAMATE DESCRIPTION
- 12. CLINICAL PHARMACOLOGY 12.1 Mechanism of Action
- 12.2 Pharmacodynamics
- 12.3 Pharmacokinetics 12.4 Special Populations 12.5 Drug-Drug Interactions
- 13. NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
- 14. CLINICAL STUDIES 14.1 Monotherapy Epilepsy Controlled Trial
- 16. HOW SUPPLIED/STORAGE AND HANDLING Topiramate tablets
- Storage and Handling
- 17. PATIENT COUNSELING INFORMATION 17.1 Eye Disorders 17.2 Oligohydrosis and Hyperthermia 17.3 Suicidal Behavior and Ideation 17.4 Metabolic Acidosis 17.5 Interference with Cognitive and Motor Performance 17.6 Hyperammonemia and Encephalopathy 17.7 Kidney
FULL PRESCRIBING INFORMATION
Enter section text here
Uses
Enter section text here
Enter section text here
Enter section text here
Enter section text here
Enter section text here
Enter section text here
Enter section text here
Enter section text here
1 INDICATIONS AND USAGE 1.1 Monotherapy Epilepsy 1.2 Adjunctive Therapy Epilepsy
1. INDICATIONS AND USAGE
1.1 Monotherapy Epilepsy
Topiramate tablets are indicated as initial monotherapy in patients 10 years of age and older with partial onset or primary generalized tonic-clonic seizures. Effectiveness was demonstrated in a controlled trial in patients with epilepsy who had no more than 2 seizures in the 3 months prior to enrollment. Safety and effectiveness in patients who were converted to monotherapy from a previous regimen of other anticonvulsant drugs have not been established in controlled trials [see Clinical Studies (14.1)].
1.2 Adjunctive Therapy Epilepsy
Topiramate tablets are indicated as adjunctive therapy for adults and pediatric patients ages 2 to 16 years with partial onset seizures or primary generalized tonic-clonic seizures, and in patients 2 years of age and older with seizures associated with Lennox-Gastaut syndrome [see Clinical Studies (14.2)].
2. DOSAGE AND ADMINISTRATION 2.1 Epilepsy 2.4 Patients with Renal Impairment 2.5 Geriatric Patients (Ages 65 Years and Over) 2.6 Patients Undergoing Hemodialysis 2.7 Patients with Hepatic Disease
2. DOSAGE AND ADMINISTRATION
2.1 Epilepsy
In the controlled adjunctive (i.e., add-on) trials, no correlation has been demonstrated between trough plasma concentrations of topiramate and clinical efficacy. No evidence of tolerance has been demonstrated in humans. Doses above 400 mg/day (600, 800 or 1,000 mg/day) have not been shown to improve responses in dose-response studies in adults with partial onset seizures.
It is not necessary to monitor topiramate plasma concentrations to optimize topiramate tablets therapy. On occasion, the addition of topiramate tablets to phenytoin may require an adjustment of the dose of phenytoin to achieve optimal clinical outcome. Addition or withdrawal of phenytoin and/or carbamazepine during adjunctive therapy with topiramate tablets may require adjustment of the dose of topiramate tablets. Because of the bitter taste, tablets should not be broken.
Topiramate tablets can be taken without regard to meals.
Monotherapy Use
The recommended dose for topiramate monotherapy in adults and pediatric patients 10 years of age and older is 400 mg/day in two divided doses. Approximately 58% of patients randomized to 400 mg/day achieved this maximal dose in the monotherapy controlled trial; the mean dose achieved in the trial was 275 mg/day. The dose should be achieved by titration according to the following schedule:
|
|
Morning Dose | Evening Dose |
| Week 1 | 25 mg | 25 mg |
| Week 2 | 50 mg | 50 mg |
| Week 3 | 75 mg | 75 mg |
| Week 4 | 100 mg | 100 mg |
| Week 5 | 150 mg | 150 mg |
| Week 6 | 200 mg | 200 mg |
Adjunctive Therapy Use
Adults (17 Years of Age and Over) - Partial Onset Seizures, Primary Generalized Tonic-Clonic Seizures, or Lennox-Gastaut Syndrome
The recommended total daily dose of topiramate tablets as adjunctive therapy in adults with partial onset seizures is 200 to 400 mg/day in two divided doses, and 400 mg/day in two divided doses as adjunctive treatment in adults with primary generalized tonic-clonic seizures. It is recommended that therapy be initiated at 25 to 50 mg/day followed by titration to an effective dose in increments of 25 to 50 mg/day every week. Titrating in increments of 25 mg/day every week may delay the time to reach an effective dose. Daily doses above 1,600 mg have not been studied.
In the study of primary generalized tonic-clonic seizures the initial titration rate was slower than in previous studies; the assigned dose was reached at the end of 8 weeks [see Clinical Studies (14.1)].
Pediatric Patients (Ages 2 - 16 Years) – Partial Onset Seizures, Primary Generalized Tonic-Clonic Seizures, or Lennox-Gastaut Syndrome
The recommended total daily dose of topiramate tablets as adjunctive therapy for pediatric patients with partial onset seizures, primary generalized tonic-clonic seizures, or seizures associated with Lennox-Gastaut syndrome is approximately 5 to 9 mg/kg/day in two divided doses. Titration should begin at 25 mg/day (or less, based on a range of 1 to 3 mg/kg/day) nightly for the first week. The dosage should then be increased at 1- or 2-week intervals by increments of 1 to 3 mg/kg/day (administered in two divided doses), to achieve optimal clinical response. Dose titration should be guided by clinical outcome.
In the study of primary generalized tonic-clonic seizures the initial titration rate was slower than in previous studies; the assigned dose of 6 mg/kg/day was reached at the end of 8 weeks [see Clinical Studies (14.1)].
2.4 Patients with Renal Impairment
In renally impaired subjects (creatinine clearance less than 70 mL/min/1.73 m2), one half of the usual adult dose is recommended. Such patients will require a longer time to reach steady-state at each dose.
2.5 Geriatric Patients (Ages 65 Years and Over)
Dosage adjustment may be indicated in the elderly patient when impaired renal function (creatinine clearance rate less than 70 mL/min/1.73 m2) is evident [see Clinical Pharmacology 12.3].
2.6 Patients Undergoing Hemodialysis
Topiramate is cleared by hemodialysis at a rate that is 4 to 6 times greater than a normal individual. Accordingly, a prolonged period of dialysis may cause topiramate concentration to fall below that required to maintain an anti-seizure effect. To avoid rapid drops in topiramate plasma concentration during hemodialysis, a supplemental dose of topiramate may be required. The actual adjustment should take into account 1) the duration of dialysis period, 2) the clearance rate of the dialysis system being used, and 3) the effective renal clearance of topiramate in the patient being dialyzed.
2.7 Patients with Hepatic Disease
In hepatically impaired patients topiramate plasma concentrations may be increased. The mechanism is not well understood.
3. DOSAGE FORMS AND STRENGTHS
3. DOSAGE FORMS AND STRENGTHS
Topiramate tablets are available as debossed, coated, round tablets in the following strengths and colors:
25 mg white to off white (debossed "1031" on one side; "25" on the other)
50 mg yellow (debossed "1032" on one side; "50" on the other)
100 mg light yellow (debossed "1033" on one side; "100" on the other)
200 mg peach (debossed "1034" on one side; "200" on the other)
4. CONTRAINDICATIONS
4. CONTRAINDICATIONS
None.
5. WARNINGS AND PRECAUTIONS 5.1 Acute Myopia and Secondary Angle Closure Glaucoma 5.2 Oligohidrosis and Hyperthermia 5.3 Suicidal Behavior and Ideation 5.4 Metabolic Acidosis 5.5 Cognitive/Neuropsychiatric Side Effects 5.6 Withdrawal of Antiepileptic
5. WARNINGS AND PRECAUTIONS
5.1 Acute Myopia and Secondary Angle Closure Glaucoma
A syndrome consisting of acute myopia associated with secondary angle closure glaucoma has been reported in patients receiving topiramate. Symptoms include acute onset of decreased visual acuity and/or ocular pain. Ophthalmologic findings can include myopia, anterior chamber shallowing, ocular hyperemia (redness) and increased intraocular pressure. Mydriasis may or may not be present. This syndrome may be associated with supraciliary effusion resulting in anterior displacement of the lens and iris, with secondary angle closure glaucoma. Symptoms typically occur within 1 month of initiating topiramate tablets therapy. In contrast to primary narrow angle glaucoma, which is rare under 40 years of age, secondary angle closure glaucoma associated with topiramate has been reported in pediatric patients as well as adults. The primary treatment to reverse symptoms is discontinuation of topiramate tablets as rapidly as possible, according to the judgment of the treating physician. Other measures, in conjunction with discontinuation of topiramate tablets, may be helpful.
Elevated intraocular pressure of any etiology, if left untreated, can lead to serious sequelae including permanent vision loss.
5.2 Oligohidrosis and Hyperthermia
Oligohidrosis (decreased sweating), infrequently resulting in hospitalization, has been reported in association with topiramate use. Decreased sweating and an elevation in body temperature above normal characterized these cases. Some of the cases were reported after exposure to elevated environmental temperatures.
The majority of the reports have been in pediatric patients. Patients, especially pediatric patients, treated with topiramate should be monitored closely for evidence of decreased sweating and increased body temperature, especially in hot weather. Caution should be used when topiramate is prescribed with other drugs that predispose patients to heat-related disorders; these drugs include, but are not limited to, other carbonic anhydrase inhibitors and drugs with anticholinergic activity.
5.3 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including topiramate, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed.
Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
Table 1 : Risk by Indication for Antiepileptic Drugs in Pooled Analysis
| Indication | Placebo Patients with Events per 1000 Patients |
Drug Patients with Events per 1000 Patients |
Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients |
Risk Difference: Additional Drug Patients with Events per 1000 Patients |
| Epilepsy | 1.0 | 3.4 | 3.5 | 2.4 |
| Psychiatric | 5.7 | 8.5 | 1.5 | 2.9 |
| Other | 1.0 | 1.8 | 1.9 | 0.9 |
| Total | 2.4 | 4.3 | 1.8 | 1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing topiramate or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior or the emergence of suicidal thoughts, behavior or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
5.4 Metabolic Acidosis
Hyperchloremic, non-anion gap, metabolic acidosis (i.e., decreased serum bicarbonate below the normal reference range in the absence of chronic respiratory alkalosis) is associated with topiramate treatment. This metabolic acidosis is caused by renal bicarbonate loss due to the inhibitory effect of topiramate on carbonic anhydrase. Such electrolyte imbalance has been observed with the use of topiramate in placebo-controlled clinical trials and in the post-marketing period. Generally, topiramate-induced metabolic acidosis occurs early in treatment although cases can occur at any time during treatment. Bicarbonate decrements are usually mild-moderate (average decrease of 4 mEq/L at daily doses of 400 mg in adults and at approximately 6 mg/kg/day in pediatric patients); rarely, patients can experience severe decrements to values below 10 mEq/L. Conditions or therapies that predispose patients to acidosis (such as renal disease, severe respiratory disorders, status epilepticus, diarrhea, ketogenic diet or specific drugs) may be additive to the bicarbonate lowering effects of topiramate.
In adults, the incidence of persistent treatment-emergent decreases in serum bicarbonate (levels of less than 20 mEq/L at two consecutive visits or at the final visit) in controlled clinical trials for adjunctive treatment of epilepsy was 32% for 400 mg/day, and 1% for placebo. Metabolic acidosis has been observed at doses as low as 50 mg/day. The incidence of persistent treatment-emergent decreases in serum bicarbonate in adults in the epilepsy controlled clinical trial for monotherapy was 15% for 50 mg/day and 25% for 400 mg/day. The incidence of a markedly abnormally low serum bicarbonate (i.e., absolute value less than 17 mEq/L and greater than 5 mEq/L decrease from pretreatment) in the adjunctive therapy trials was 3% for 400 mg/day, and 0% for placebo and in the monotherapy trial was 1% for 50 mg/day and 7% for 400 mg/day. Serum bicarbonate levels have not been systematically evaluated at daily doses greater than 400 mg/day.
In pediatric patients (2-16 years of age), the incidence of persistent treatment-emergent decreases in serum bicarbonate in placebo-controlled trials for adjunctive treatment of Lennox-Gastaut syndrome or refractory partial onset seizures was 67% for topiramate (at approximately 6 mg/kg/day), and 10% for placebo. The incidence of a markedly abnormally low serum bicarbonate (i.e., absolute value less than 17 mEq/L and greater than 5 mEq/L decrease from pretreatment) in these trials was 11% for topiramate and 0% for placebo. Cases of moderately severe metabolic acidosis have been reported in patients as young as 5 months old, especially at daily doses above 5 mg/kg/day.
Although not approved for use in patients under 2 years of age with partial onset seizures, a controlled trial that examined this population revealed that topiramate produced a metabolic acidosis that is notably greater in magnitude than that observed in controlled trials in older children and adults. The mean treatment difference (25 mg/kg/d topiramate-placebo) was -5.9 mEq/L for bicarbonate. The incidence of metabolic acidosis (defined by a serum bicarbonate less than 20 mEq/L) was 0% for placebo, 30% for 5 mg/kg/d, 50% for 15 mg/kg/d, and 45% for 25 mg/kg/d [see Pediatric Use (8.4)].
In pediatric patients (10 years up to 16 years of age), the incidence of persistent treatment-emergent decreases in serum bicarbonate in the epilepsy controlled clinical trial for monotherapy was 7% for 50 mg/day and 20% for 400 mg/day. The incidence of a markedly abnormally low serum bicarbonate (i.e., absolute value less than 17 mEq/L and greater than 5 mEq/L decrease from pretreatment) in this trial was 4% for 50 mg/day and 4% for 400 mg/day. The incidence of persistent treatment-emergent decreases in serum bicarbonate in placebo-controlled trials for adults for another indication was 44% for 200 mg/day, 39% for 100 mg/day, 23% for 50 mg/day, and 7% for placebo. The incidence of a markedly abnormally low serum bicarbonate (i.e., absolute value less than 17 mEq/L and greater than 5 mEq/L decrease from pretreatment) in these trials was 11% for 200 mg/day, 9% for 100 mg/day, 2% for 50 mg/day, and less than 1% for placebo.
Some manifestations of acute or chronic metabolic acidosis may include hyperventilation, nonspecific symptoms such as fatigue and anorexia, or more severe sequelae including cardiac arrhythmias or stupor. Chronic, untreated metabolic acidosis may increase the risk for nephrolithiasis or nephrocalcinosis, and may also result in osteomalacia (referred to as rickets in pediatric patients) and/or osteoporosis with an increased risk for fractures. Chronic metabolic acidosis in pediatric patients may also reduce growth rates. A reduction in growth rate may eventually decrease the maximal height achieved. The effect of topiramate on growth and bone-related sequelae has not been systematically investigated in long-term, placebo-controlled trials. Long-term, open-label treatment of infants/toddlers, with intractable partial epilepsy, for up to 1 year, showed reductions from baseline in Z SCORES for length, weight, and head circumference compared to age and sex-matched normative data, although these patients with epilepsy are likely to have different growth rates than normal infants. Reductions in Z SCORES for length and weight were correlated to the degree of acidosis [see Pediatric Use (8.4)].
Measurement of baseline and periodic serum bicarbonate during topiramate treatment is recommended. If metabolic acidosis develops and persists, consideration should be given to reducing the dose or discontinuing topiramate (using dose tapering). If the decision is made to continue patients on topiramate in the face of persistent acidosis, alkali treatment should be considered.
5.5 Cognitive/Neuropsychiatric Adverse Reactions
Adverse reactions most often associated with the use of topiramate were related to the central nervous system and were observed in both the epilepsy and other populations. In adults, the most frequent of these can be classified into three general categories: 1) Cognitive-related dysfunction (e.g. confusion, psychomotor slowing, difficulty with concentration/attention, difficulty with memory, speech or language problems, particularly word-finding difficulties); 2) Psychiatric/behavioral disturbances (e.g. depression or mood problems); and 3) Somnolence or fatigue.
Adult Patients
Cognitive-Related Dysfunction
The majority of cognitive-related adverse reactions were mild to moderate in severity, and they frequently occurred in isolation. Rapid titration rate and higher initial dose were associated with higher incidences of these reactions. Many of these reactions contributed to withdrawal from treatment [see Adverse Reactions (6)].
In the add-on epilepsy controlled trials (using rapid titration such as 100-200 mg/day weekly increments), the proportion of patients who experienced one or more cognitive-related adverse reactions was 42% for 200 mg/day, 41% for 400 mg/day, 52% for 600 mg/day, 56% for 800 and 1000 mg/day, and 14% for placebo. These dose-related adverse reactions began with a similar frequency in the titration or in the maintenance phase, although in some patients the events began during titration and persisted into the maintenance phase. Some patients who experienced one or more cognitive-related adverse reactions in the titration phase had a dose-related recurrence of these reactions in the maintenance phase.
In the monotherapy epilepsy controlled trial, the proportion of patients who experienced one or more cognitive-related adverse reactions was 19% for topiramate 50 mg/day and 26% for 400 mg/day.
In the 6-month controlled trials for another indication using a slower titration regimen (25 mg/day weekly increments), the proportion of patients who experienced one or more cognitive-related adverse reactions was 19% for topiramate 50 mg/day, 22% for 100 mg/day (the recommended dose), 28% for 200 mg/day, and 10% for placebo. These dose-related adverse reactions typically began in the titration phase and often persisted into the maintenance phase, but infrequently began in the maintenance phase. Some patients experienced a recurrence of one or more of these cognitive adverse reactions and this recurrence was typically in the titration phase. A relatively small proportion of topiramate-treated patients experienced more than one concurrent cognitive adverse reaction. The most common cognitive adverse reactions occurring together included difficulty with memory along with difficulty with concentration/attention, difficulty with memory along with language problems, and difficulty with concentration/attention along with language problems. Rarely, topiramate-treated patients experienced three concurrent cognitive reactions.
Psychiatric/Behavioral Disturbances
Psychiatric/behavioral disturbances (depression or mood) were dose-related for both the epilepsy and other populations [see Warnings and Precautions (5.3)].
Somnolence/Fatigue
Somnolence and fatigue were the adverse reactions most frequently reported during clinical trials of topiramate for adjunctive epilepsy. For the adjunctive epilepsy population, the incidence of somnolence did not differ substantially between 200 mg/day and 1000 mg/day, but the incidence of fatigue was dose-related and increased at dosages above 400 mg/day. For the monotherapy epilepsy population in the 50 mg/day and 400 mg/day groups, the incidence of somnolence was dose-related (9% for the 50 mg/day group and 15% for the 400 mg/day group) and the incidence of fatigue was comparable in both treatment groups (14% each). For another population, fatigue and somnolence were dose-related and more common in the titration phase.
Additional nonspecific CNS events commonly observed with topiramate in the add-on epilepsy population include dizziness or ataxia.
Pediatric Patients
In double-blind adjunctive therapy and monotherapy epilepsy clinical studies, the incidences of cognitive/neuropsychiatric adverse reactions in pediatric patients were generally lower than observed in adults. These reactions included psychomotor slowing, difficulty with concentration/attention, speech disorders/related speech problems and language problems. The most frequently reported neuropsychiatric reactions in pediatric patients during adjunctive therapy double-blind studies were somnolence and fatigue. The most frequently reported neuropsychiatric reactions in pediatric patients in the 50 mg/day and 400 mg/day groups during the monotherapy double-blind study were headache, dizziness, anorexia, and somnolence.
No patients discontinued treatment due to any adverse events in the adjunctive epilepsy double-blind trials. In the monotherapy epilepsy double-blind trial, 1 pediatric patient (2%) in the 50 mg/day group and 7 pediatric patients (12%) in the 400 mg/day group discontinued treatment due to any adverse events. The most common adverse reaction associated with discontinuation of therapy was difficulty with concentration/attention; all occurred in the 400 mg/day group.
5.6 Withdrawal of Antiepileptic Drugs (AEDs)
In patients with or without a history of seizures or epilepsy, antiepileptic drugs including topiramate should be gradually withdrawn to minimize the potential for seizures or increased seizure frequency [see Clinical Studies (14)]. In situations where rapid withdrawal of topiramate is medically required, appropriate monitoring is recommended.
5.7 Sudden Unexplained Death in Epilepsy (SUDEP)
During the course of premarketing development of topiramate tablets, 10 sudden and unexplained deaths were recorded among a cohort of treated patients (2,796 subject years of exposure). This represents an incidence of 0.0035 deaths per patient year. Although this rate exceeds that expected in a healthy population matched for age and sex, it is within the range of estimates for the incidence of sudden unexplained deaths in patients with epilepsy not receiving topiramate (ranging from 0.0005 for the general population of patients with epilepsy, to 0.003 for a clinical trial population similar to that in the topiramate program, to 0.005 for patients with refractory epilepsy).
5.8 Hyperammonemia and Encephalopathy (Without and With Concomitant Valproic Acid [VPA] Use) Hyperammonemia/Encephalopathy Without Concomitant Valproic Acid (VPA)
Hyperammonemia/Encephalopathy Without Concomitant Valproic Acid (VPA)
Topiramate treatment has produced hyperammonemia (in some instances dose-related) in clinical investigational programs of adolescents (12-16 years) who were treated with topiramate monotherapy for another indication (incidence above normal, 22% for placebo, 26 % for 50 mg/day, 41% for 100 mg daily) and in very young pediatric patients (1-24 months) who were treated with adjunctive topiramate for partial onset epilepsy (8% for placebo, 10 % for 5 mg/kg/day, 0 % for 15 mg/kg/day, 9 % for 25 mg/kg/day). Topiramate is not approved as monotherapy for another indication in adolescent patients or as adjunctive treatment of partial onset seizures in pediatric patients less than 2 years old. In some patients, ammonia was markedly increased (greater than 50 % above upper limit of normal). In the adolescent patients, the incidence of markedly increased hyperammonemia was 6 % for placebo, 6 % for 50 mg, and 12 % for 100 mg topiramate daily. The hyperammonemia associated with topiramate treatment occurred with and without encephalopathy in placebo-controlled trials, and in an open-label, extension trial. Dose-related hyperammonemia was also observed in the extension trial in pediatric patients up to 2 years old. Clinical symptoms of hyperammonemic encephalopathy often include acute alterations in level of consciousness and/or cognitive function with lethargy or vomiting.
Hyperammonemia with and without encephalopathy has also been observed in post-marketing reports in patients who were taking topiramate without concomitant valproic acid (VPA).
Hyperammonemia/Encephalopathy With Concomitant Valproic Acid (VPA)
Concomitant administration of topiramate and valproic acid (VPA) has been associated with hyperammonemia with or without encephalopathy in patients who have tolerated either drug alone based upon post-marketing reports. Although hyperammonemia may be asymptomatic, clinical symptoms of hyperammonemic encephalopathy often include acute alterations in level of consciousness and/or cognitive function with lethargy or vomiting. In most cases, symptoms and signs abated with discontinuation of either drug. This adverse reaction is not due to a pharmacokinetic interaction.
Although topiramate is not indicated for use in infants/toddlers (1-24 months) VPA clearly produced a dose-related increased in the incidence of treatment-emergent hyperammonemia (above the upper limit of normal, 0% for placebo, 12% for 5 mg/kg/day, 7% for 15 mg/kg/day, 17% for 25 mg/kg/day) in an investigational program. Markedly increased, dose-related hyperammonemia (0% for placebo and 5 mg/kg/day, 7% for 15 mg/kg/day, 8 % for 25 mg/kg/day) also occurred in these infants/toddlers. Dose-related hyperammonemia was similarly observed in a long-term, extension trial in these very young, pediatric patients [see Use in Specific Populations (8.4)].
Hyperammonemia with and without encephalopathy has also been observed in post-marketing reports in patients taking topiramate with valproic acid (VPA).
The hyperammonemia associated with topiramate treatment appears to be more common when topiramate is used concomitantly with VPA.
Monitoring for Hyperammonemia
Patients with inborn errors of metabolism or reduced hepatic mitochondrial activity may be at an increased risk for hyperammonemia with or without encephalopathy. Although not studied, topiramate treatment or an interaction of concomitant topiramate and valproic acid treatment may exacerbate existing defects or unmask deficiencies in susceptible persons.
In patients who develop unexplained lethargy, vomiting, or changes in mental status associated with any topiramate treatment, hyperammonemic encephalopathy should be considered and an ammonia level should be measured.
5.9 Kidney Stones
A total of 32/2,086 (1.5%) of adults exposed to topiramate during its adjunctive epilepsy therapy development reported the occurrence of kidney stones, an incidence about 2 to 4 times greater than expected in a similar, untreated population. In the double-blind monotherapy epilepsy study, a total of 4/319 (1.3%) of adults exposed to topiramate reported the occurrence of kidney stones. As in the general population, the incidence of stone formation among topiramate treated patients was higher in men. Kidney stones have also been reported in pediatric patients. During long-term (up to 1 year) topiramate treatment in an open-label extension study of 284 pediatric patients 1-24 months old with epilepsy, 7% developed kidney or bladder stones that were diagnosed clinically or by sonogram. Topiramate is not approved for pediatric patients less than 2 years old [see Pediatric Use (8.4)].
An explanation for the association of topiramate and kidney stones may lie in the fact that topiramate is a carbonic anhydrase inhibitor. Carbonic anhydrase inhibitors (e.g., zonisamide, acetazolamide or dichlorphenamide) can promote stone formation by reducing urinary citrate excretion and by increasing urinary pH [see Warnings and Precautions (5.4)]. The concomitant use of topiramate with any other drug producing metabolic acidosis, or potentially in patients on a ketogenic diet may create a physiological environment that increases the risk of kidney stone formation, and should therefore be avoided.
Increased fluid intake increases the urinary output, lowering the concentration of substances involved in stone formation. Hydration is recommended to reduce new stone formation.
5.10 Paresthesia
Paresthesia (usually tingling of the extremities), an effect associated with the use of other carbonic anhydrase inhibitors, appears to be a common effect of topiramate. Paresthesia was more frequently reported in the monotherapy epilepsy trials and trials for another indication than in the adjunctive therapy epilepsy trials. In the majority of instances, paresthesia did not lead to treatment discontinuation.
5.11 Adjustment of Dose in Renal Failure
The major route of elimination of unchanged topiramate and its metabolites is via the kidney. Dosage adjustment may be required in patients with reduced renal function [see Dosage and Administration (2)].
5.12 Decreased Hepatic Function
In hepatically impaired patients, topiramate should be administered with caution as the clearance of topiramate may be decreased.
5.13 Monitoring: Laboratory Tests
Topiramate treatment was associated with changes in several clinical laboratory analytes in randomized, double-blind, placebo-controlled studies.
Topiramate treatment causes non-anion gap, hyperchloremic, metabolic acidosis manifested by a decrease in serum bicarbonate and increase in serum chloride. Measurement of baseline and periodic serum bicarbonate during topiramate treatment is recommended [see Warnings and Precautions (5.4)].
Controlled trials of adjunctive topiramate treatment of adults for partial onset seizures showed an increased incidence of markedly decreased serum phosphorus (6% topiramate, 2% placebo), markedly increased serum alkaline phosphatase (3% topiramate, 1% placebo), and decreased serum potassium (0.4 % topiramate, 0.1 % placebo). The clinical significance of these abnormalities has not been clearly established.
Changes in several clinical laboratory laboratories (increased creatinine, BUN, alkaline phosphatase, total protein, total eosinophil count and decreased potassium) have been observed in a clinical investigational program in very young (less than 2 years) pediatric patients who were treated with adjunctive topiramate for partial onset seizures [see Pediatric Use (8.4)].
Topiramate treatment produced a dose-related increased shift in serum creatinine from normal at baseline to an increased value at the end of 4 months treatment in adolescent patients (ages 12-16 years) who were treated for another indication in a double-blind, placebo-controlled study. Topiramate treatment with or without concomitant valproic acid (VPA) can cause hyperammonemia with or without encephalopathy [see Warnings and Precautions (5.8)].
Table 2 Table 3 Table 4 Table 6 Table 7 6. ADVERSE REACTIONS 6.1 Monotherapy Epilepsy 6.2 Adjunctive Therapy Epilepsy 6.3 Incidence in Epilepsy Controlled Clinical Trials – Adjunctive Therapy – Partial Onset Seizures, Primary Generalized Tonic-Clonic Seiz
6. ADVERSE REACTIONS
The data described in the following section were obtained using topiramate tablets.
6.1 Monotherapy Epilepsy
The adverse reactions in the controlled trial that occurred most commonly in adults in the 400 mg/day group and at a rate higher than the 50 mg/day group were: paresthesia, weight decrease, somnolence, anorexia, dizziness, and difficulty with memory NOS [see Table 2 ].
The adverse reactions in the controlled trial that occurred most commonly in children (10 years up to 16 years of age) in the 400 mg/day group and at a rate higher than the 50 mg/day group were: weight decrease, upper respiratory tract infection, paresthesia, anorexia, diarrhea, and mood problems [see Table 3 ].
Approximately 21% of the 159 adult patients in the 400 mg/day group who received topiramate as monotherapy in the controlled clinical trial discontinued therapy due to adverse reactions. Adverse reactions associated with discontinuing therapy (greater than 2%) included depression, insomnia, difficulty with memory (NOS), somnolence, paresthesia, psychomotor slowing, dizziness, and nausea.
Approximately 12% of the 57 pediatric patients in the 400 mg/day group who received topiramate as monotherapy in the controlled clinical trial discontinued therapy due to adverse reactions. Adverse reactions associated with discontinuing therapy (greater than 5%) included difficulty with concentration/attention.
The prescriber should be aware that these data cannot be used to predict the frequency of adverse reactions in the course of usual medical practice where patient characteristics and other factors may differ from those prevailing during the clinical study. Similarly, the cited frequencies cannot be directly compared with data obtained from other clinical investigations involving different treatments, uses, or investigators. Inspection of these frequencies, however, does provide the prescribing physician with a basis to estimate the relative contribution of drug and non-drug factors to the adverse reactions incidences in the population studied.
Table 2: Incidence of Treatment-Emergent Adverse Reaction in the Monotherapy Epilepsy Trial in Adultsa Where Incidence Was at Least 2% in the 400 mg/day Topiramate Group and Greater Than the Rate in the 50 mg/day Topiramate Group
|
|
Topiramate Tablets Dosage (mg/day) |
|
| Body System/ Adverse Reaction |
50 (N=160) |
400 (N=159) |
| Body as a Whole - General Disorders |
|
|
| Asthenia | 4 |
6 |
| Leg Pain | 2 |
3 |
| Chest Pain | 1 |
2 |
| Central and Peripheral Nervous System Disorders |
|
|
| Paresthesia |
21 |
40 |
| Dizziness |
13 |
14 |
| Hypoaesthesia | 4 |
5 |
| Ataxia | 3 |
4 |
| Hypertonia |
0 |
3 |
| Gastro-Intestinal System Disorders |
|
|
| Diarrhea |
5 |
6 |
| Constipation |
1 |
4 |
| Gastritis | 0 |
3 |
| Dry Mouth | 1 |
3 |
| Gastroesophageal Reflux | 1 |
2 |
| Liver and Biliary System Disorders |
|
|
| Gamma-GT Increased | 1 |
3 |
| Metabolic and Nutritional Disorders |
|
|
| Weight Decrease |
6 |
16 |
| Psychiatric Disorders |
|
|
| Somnolence | 9 |
15 |
| Anorexia | 4 |
14 |
| Difficulty with Memory NOS | 5 |
10 |
| Insomnia | 8 |
9 |
| Depression | 7 |
9 |
| Difficulty with Concentration/Attention | 7 |
8 |
| Anxiety | 4 |
6 |
| Psychomotor Slowing | 3 |
5 |
| Mood Problems | 2 |
5 |
| Confusion | 3 |
4 |
| Cognitive Problem NOS | 1 |
4 |
| Libido Decreased | 0 |
3 |
| Reproductive Disorders, Female |
|
|
| Vaginal Hemorrhage | 0 |
3 |
| Red Blood Cell Disorders |
|
|
| Anemia | 1 |
2 |
| Resistance Mechanism Disorders |
|
|
| Infection Viral |
6 |
8 |
| Infection | 2 |
3 |
| Respiratory System Disorders |
|
|
| Bronchitis | 3 |
4 |
| Rhinitis | 2 |
4 |
| Dyspnea |
1 |
2 |
| Skin and Appendages Disorders |
|
|
| Rash | 1 |
4 |
| Pruritus |
1 |
4 |
| Acne | 2 |
3 |
| Special Senses Other, Disorders |
|
|
| Taste Perversion | 3 |
5 |
| Urinary System Disorders |
|
|
| Cystitis | 1 |
3 |
| Renal Calculus | 0 |
3 |
| Urinary Tract Infection | 1 |
2 |
| Dysuria |
0 |
2 |
| Micturition Frequency | 0 |
2 |
| a Values represent the percentage of patients reporting a given adverse reaction. Patients may have reported more than one adverse reaction during the study and can be included in more than one adverse reaction category. |
|
|
|
|
Topiramate Tablets Dosage (mg/day) |
|
| Body System/ Adverse Reaction |
50 (N=57) |
400 (N=57) |
| Body as a Whole-General Disorders |
|
|
| Fever |
0 |
9 |
| Central and Peripheral Nervous System Disorders |
|
|
| Paresthesia |
2 |
16 |
| Gastro-Intestinal System Disorders |
|
|
| Diarrhea |
5 |
11 |
| Metabolic and Nutritional Disorders |
|
|
| Weight Decrease |
7 |
21 |
| Psychiatric Disorders |
|
|
| Anorexia |
11 |
14 |
| Mood Problems |
2 |
11 |
| Difficulty with Concentration/Attention |
4 |
9 |
| Cognitive Problems NOS |
0 |
7 |
| Nervousness |
4 |
5 |
| Resistance Mechanism Disorders |
|
|
| Infection Viral |
4 |
9 |
| Infection |
2 |
7 |
| Respiratory System Disorders |
|
|
| Upper Respiratory Tract Infection |
16 |
18 |
| Rhinitis |
2 |
7 |
| Bronchitis |
2 |
7 |
| Sinusitis |
2 |
5 |
| Skin and Appendages Disorders |
|
|
| Alopecia |
2 |
5 |
| a Values represent the percentage of patients reporting a given adverse reaction. Patients may have reported more than one adverse reaction during the study and can be included in more than one adverse reaction category. |
|
|
Table 4Table 6
Table 7
|
|
Topiramate Tablets Dosage (mg/day) |
|
|
| Body System/ Adverse Reaction c |
Placebo (N=291) |
200-400 (N=183) |
600-1,000 (N=414) |
| Body as a Whole-General Disorders |
|
|
|
| Fatigue |
13 |
15 |
30 |
| Asthenia |
1 |
6 |
3 |
| Back Pain |
4 |
5 |
3 |
| Chest Pain |
3 |
4 |
2 |
| Influenza-Like Symptoms |
2 |
3 |
4 |
| Leg Pain |
2 |
2 |
4 |
| Hot Flushes |
1 |
2 |
1 |
| Allergy |
1 |
2 |
3 |
| Edema |
1 |
2 |
1 |
| Body Odor |
0 |
1 |
0 |
| Rigors |
0 |
1 |
less than 1 |
| Central and Peripheral Nervous System Disorders |
|
|
|
| Dizziness |
15 |
25 |
32 |
| Ataxia |
7 |
16 |
14 |
| Speech Disorders/Related Speech Problems |
2 |
13 |
11 |
| Paresthesia |
4 |
11 |
19 |
| Nystagmus |
7 |
10 |
11 |
| Tremor |
6 |
9 |
9 |
| Language Problems |
1 |
6 |
10 |
| Coordination Abnormal |
2 |
4 |
4 |
| Hypoaesthesia |
1 |
2 |
1 |
| Gait Abnormal |
1 |
3 |
2 |
| Muscle Contractions Involuntary |
1 |
2 |
2 |
| Stupor |
0 |
2 |
1 |
| Vertigo |
1 |
1 |
2 |
| Gastro-Intestinal System Disorders |
|
|
|
| Nausea |
8 |
10 |
12 |
| Dyspepsia |
6 |
7 |
6 |
| Abdominal Pain |
4 |
6 |
7 |
| Constipation |
2 |
4 |
3 |
| Gastroenteritis |
1 |
2 |
1 |
| Dry Mouth |
1 |
2 |
4 |
| Gingivitis |
less than 1 |
1 |
1 |
| GI Disorder |
less than 1 |
1 |
0 |
| Hearing and Vestibular Disorders |
|
|
|
| Hearing Decreased |
1 |
2 |
1 |
| Metabolic and Nutritional Disorders |
|
|
|
| Weight Decrease |
3 |
9 |
13 |
| Muscle-Skeletal System Disorders |
|
|
|
| Myalgia |
1 |
2 |
2 |
| Skeletal pain |
0 |
1 |
0 |
| Platelet, Bleeding, and Clotting Disorders |
|
|
|
| Epistaxis |
1 |
2 |
1 |
| Psychiatric Disorders |
|
|
|
| Somnolence |
12 |
29 |
28 |
| Nervousness |
6 |
16 |
19 |
| Psychomotor Slowing |
2 |
13 |
21 |
| Difficulty with Memory |
3 |
12 |
14 |
| Anorexia |
4 |
10 |
12 |
| Confusion |
5 |
11 |
14 |
| Depression |
5 |
5 |
13 |
| Difficulty with Concentration/Attention |
2 |
6 |
14 |
| Mood Problems |
2 |
4 |
9 |
| Agitation |
2 |
3 |
3 |
| Aggressive Reaction |
2 |
3 |
3 |
| Emotional Lability |
1 |
3 |
3 |
| Cognitive Problems |
1 |
3 |
3 |
| Libido Decreased |
1 |
2 |
less than 1 |
| Apathy |
1 |
1 |
3 |
| Depersonalization |
1 |
1 |
2 |
| Reproductive Disorders, Female |
|
|
|
| Breast Pain |
2 |
4 |
0 |
| Amenorrhea |
1 |
2 |
2 |
| Menorrhagia |
0 |
2 |
1 |
| Menstrual Disorder | 1 |
2 |
1 |
| Reproductive Disorders, Male |
|
|
|
| Prostatic Disorder |
less than 1 |
2 |
0 |
| Resistance Mechanism Disorders |
|
|
|
| Infection |
1 |
2 |
1 |
| Infection Viral |
1 |
2 |
less than 1 |
| Moniliasis |
less than 1 |
1 |
0 |
| Respiratory System Disorders |
|
|
|
| Pharyngitis |
2 |
6 |
3 |
| Rhinitis |
6 |
7 |
6 |
| Sinusitis |
4 |
5 |
6 |
| Dyspnea |
1 |
1 |
2 |
| Skin and Appendages Disorders |
|
|
|
| Skin Disorder |
less than 1 |
2 |
1 |
| Sweating Increased |
less than 1 |
1 |
less than 1 |
| Rash Erythematous |
less than 1 |
1 |
less than 1 |
| Special Sense Other, Disorders |
|
|
|
| Taste Perversion |
0 |
2 |
4 |
| Urinary System Disorders |
|
|
|
| Hematuria |
1 |
2 |
less than 1 |
| Urinary Tract Infection |
1 |
2 |
3 |
| Micturition Frequency |
1 |
1 |
2 |
| Urinary Incontinence |
less than 1 |
2 |
1 |
| Urine Abnormal |
0 |
1 |
less than 1 |
| Vision Disorders |
|
|
|
| Vision Abnormal |
2 |
13 |
10 |
| Diplopia |
5 |
10 |
10 |
| White Cell and RES Disorders |
|
|
|
| Leukopenia |
1 |
2 |
1 |
|
|
|
Topiramate Tablets Dosage (mg/day) |
| Body System/ Adverse Reaction c |
Placebo (N=92) |
200 (N=171) |
| Body as a Whole-General Disorders |
|
|
| Fatigue |
4 |
9 |
| Chest Pain |
1 |
2 |
| Cardiovascular Disorders, General |
|
|
| Hypertension |
0 |
2 |
| Central and Peripheral Nervous System Disorders |
|
|
| Paresthesia |
2 |
9 |
| Dizziness |
4 |
7 |
| Tremor |
2 |
3 |
| Hypoasthesia |
0 |
2 |
| Leg Cramps |
0 |
2 |
| Language Problems |
0 |
2 |
| Gastro-Intestinal System Disorders |
|
|
| Abdominal Pain |
3 |
5 |
| Constipation |
0 |
4 |
| Diarrhea |
1 |
2 |
| Dyspepsia |
0 |
2 |
| Dry Mouth |
0 |
2 |
| Hearing and Vestibular Disorders |
|
|
| Tinnitus |
0 |
2 |
| Metabolic and Nutritional Disorders |
|
|
| Weight Decrease |
4 |
8 |
| Psychiatric Disorders |
|
|
| Somnolence |
9 |
15 |
| Anorexia |
7 |
9 |
| Nervousness |
2 |
9 |
| Difficulty with Concentration/Attention |
0 |
5 |
| Insomnia |
3 |
4 |
| Difficulty with Memory |
1 |
2 |
| Aggressive Reaction |
0 |
2 |
| Respiratory System Disorders |
|
|
| Rhinitis |
0 |
4 |
| Urinary System Disorders |
|
|
| Cystitis |
0 |
2 |
| Vision Disorders |
|
|
| Diplopia |
0 |
2 |
| Vision Abnormal |
0 |
2 |
|
|
|
Topiramate Tablets Dosage (mg/day) |
|
|
| Adverse Reaction |
Placebo (N=216) |
200 (N=45) |
400 (N=68) |
600-1,000 (N=414) |
| Fatigue |
13 |
11 |
12 |
30 |
| Nervousness |
7 |
13 |
18 |
19 |
| Difficulty with Concentration/Attention |
1 |
7 |
9 |
14 |
| Confusion |
4 |
9 |
10 |
14 |
| Depression |
6 |
9 |
7 |
13 |
| Anorexia |
4 |
4 |
6 |
12 |
| Language Problems |
less than 1 |
2 |
9 |
10 |
| Anxiety |
6 |
2 |
3 |
10 |
| Mood problems |
2 |
0 |
6 |
9 |
| Weight decrease |
3 |
4 |
9 |
13 |
| Body System/ Adverse Reaction |
Placebo (N=101) |
Topiramate (N=98) |
| Body as a Whole - General Disorders |
|
|
| Fatigue |
5 |
16 |
| Injury |
13 |
14 |
| Allergic Reaction |
1 |
2 |
| Back Pain |
0 |
1 |
| Pallor |
0 |
1 |
| Cardiovascular Disorders, General |
|
|
| Hypertension |
0 |
1 |
| Central and Peripheral Nervous System Disorders |
|
|
| Gait Abnormal |
5 |
8 |
| Ataxia |
2 |
6 |
| Hyperkinesia |
4 |
5 |
| Dizziness |
2 |
4 |
| Speech Disorders/Related Speech Problems |
2 |
4 |
| Hyporeflexia |
0 |
2 |
| Convulsions Grand Mal |
0 |
1 |
| Fecal Incontinence |
0 |
1 |
| Paresthesia |
0 |
1 |
| Gastro-Intestinal System Disorders |
|
|
| Nausea |
5 |
6 |
| Saliva Increased |
4 |
6 |
| Constipation |
4 |
5 |
| Gastroenteritis |
2 |
3 |
| Dysphagia |
0 |
1 |
| Flatulence |
0 |
1 |
| Gastroesophageal Reflux |
0 |
1 |
| Glossitis |
0 |
1 |
| Gum Hyperplasia |
0 |
1 |
| Heart Rate and Rhythm Disorders |
|
|
| Bradycardia |
0 |
1 |
| Metabolic and Nutritional Disorders |
|
|
| Weight Decrease |
1 |
9 |
| Thirst |
1 |
2 |
| Hypoglycemia |
0 |
1 |
| Weight Increase |
0 |
1 |
| Platelet, Bleeding, and Clotting Disorders |
|
|
| Purpura |
4 |
8 |
| Epistaxis |
1 |
4 |
| Hematoma |
0 |
1 |
| Prothrombin Increased |
0 |
1 |
| Thrombocytopenia |
0 |
1 |
| Psychiatric Disorders |
|
|
| Somnolence |
16 |
26 |
| Anorexia |
15 |
24 |
| Nervousness |
7 |
14 |
| Personality Disorders (Behavior Problems) |
9 |
11 |
| Difficulty with Concentration/Attention |
2 |
10 |
| Aggressive Reaction |
4 |
9 |
| Insomnia |
7 |
8 |
| Difficulty with Memory NOS |
0 |
5 |
| Confusion |
3 |
4 |
| Psychomotor Slowing |
2 |
3 |
| Appetite Increased |
0 |
1 |
| Neurosis |
0 |
1 |
| Reproductive Disorders, Female |
|
|
| Leukorrhoea |
0 |
2 |
| Resistance Mechanism Disorders |
|
|
| Infection Viral |
3 |
7 |
| Respiratory System Disorders |
|
|
| Pneumonia |
1 |
5 |
| Respiratory Disorder |
0 |
1 |
| Skin and Appendages Disorders |
|
|
| Skin Disorder |
2 |
3 |
| Alopecia |
1 |
2 |
| Dermatitis |
0 |
2 |
| Hypertrichosis |
1 |
2 |
| Rash Erythematous |
0 |
2 |
| Eczema |
0 |
1 |
| Seborrhoea |
0 |
1 |
| Skin Discoloration |
0 |
1 |
| Urinary System Disorders |
|
|
| Urinary Incontinence |
2 |
4 |
| Nocturia |
0 |
1 |
| Vision Disorders |
|
|
| Eye Abnormality |
1 |
2 |
| Vision Abnormal |
1 |
2 |
| Diplopia |
0 |
1 |
| Lacrimation Abnormal |
0 |
1 |
| Myopia |
0 |
1 |
| White Cell and RES Disorders |
|
|
| Leukopenia |
0 |
2 |
7. DRUG INTERACTIONS 7.1 Antiepileptic Drugs 7.2 CNS Depressants 7.3 Oral Contraceptives 7.4 Metformin 7.5 Lithium 7.6 Other Carbonic Anhydrase Inhibitors
7. DRUG INTERACTIONS
In vitro studies indicate that topiramate does not inhibit enzyme activity for CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2D6, CYP2E1, and CYP3A4/5 isozymes. In vitro studies indicate that topiramate is a mild inhibitor of CYP2C19 and a mild inducer of CYP3A4. Drug interactions with some antiepileptic drugs, CNS depressants and oral contraceptives are described here. For other drug interactions, please refer to Clinical Pharmacology (12.5).
7.1 Antiepileptic Drugs
Potential interactions between topiramate and standard AEDs were assessed in controlled clinical pharmacokinetic studies in patients with epilepsy. Concomitant administration of phenytoin or carbamazepine with topiramate decreased plasma concentrations of topiramate by 48% and 40% respectively when compared to topiramate given alone [Clinical Pharmacology (12.5)]. In addition, concomitant administration of valproic acid and topiramate has been associated with hyperammonemia with and without encephalopathy [see Warnings and Precautions (5.8) or Clinical Pharmacology (12.5)].
7.2 CNS Depressants
Concomitant administration of topiramate and alcohol or other CNS depressant drugs has not been evaluated in clinical studies. Because of the potential of topiramate to cause CNS depression, as well as other cognitive and/or neuropsychiatric adverse events, topiramate should be used with extreme caution if used in combination with alcohol and other CNS depressants.
7.3 Oral Contraceptives
Exposure to ethinyl estradiol was statistically significantly decreased at doses of 200, 400, and 800 mg/day (18%, 21%, and 30%, respectively) when topiramate was given as adjunctive therapy in patients taking valproic acid). However, norethindrone exposure was not significantly affected. In another pharmacokinetic interaction study in healthy volunteers with a concomitantly administered combination oral contraceptive product containing 1 mg norethindrone (NET) plus 35 mcg ethinyl estradiol (EE), topiramate, given in the absence of other medications at doses of 50 to 200 mg/day, was not associated with statistically significant changes in mean exposure (AUC) to either component of the oral contraceptive. The possibility of decreased contraceptive efficacy and increased breakthrough bleeding should be considered in patients taking combination oral contraceptive products with topiramate. Patients taking estrogen-containing contraceptives should be asked to report any change in their bleeding patterns. Contraceptive efficacy can be decreased even in the absence of breakthrough bleeding [see Clinical Pharmacology (12.5)].
7.4 Metformin
Topiramate treatment can frequently cause metabolic acidosis, a condition for which the use of metformin is contraindicated [see Clinical Pharmacology (12.5)].
7.5 Lithium
In patients, lithium levels were unaffected during treatment with topiramate at doses of 200 mg/day; however, there was an observed increase in systemic exposure of lithium (27% for Cmax and 26% for AUC) following topiramate doses of up to 600 mg/day. Lithium levels should be monitored when co-administered with high-dose topiramate [see Clinical Pharmacology (12.5)].
7.6 Other Carbonic Anhydrase Inhibitors
Concomitant use of topiramate, a carbonic anhydrase inhibitor, with any other carbonic anhydrase inhibitor (e.g., zonisamide, acetazolamide or dichlorphenamide), may increase the severity of metabolic acidosis and may also increase the risk of kidney stone formation. Therefore, if topiramate is given concomitantly with another carbonic anhydrase inhibitor, the patient should be monitored for the appearance or worsening of metabolic acidosis [see Clinical Pharmacology (12.5)].
8. USE IN SPECIFIC POPULATIONS 8.1 Pregnancy
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C.
Topiramate may cause serious adverse fetal effects, based on clinical and nonclinical data.
Topiramate treatment is associated with metabolic acidosis [see Warnings and Precautions (5.4)]. The effect of topiramate-induced metabolic acidosis has not been studied in pregnancy; however, metabolic acidosis in pregnancy (due to other causes) may be associated with decreased fetal growth, decreased fetal oxygenation, and fetal death, and may affect the fetus’ ability to tolerate labor. Pregnant patients should be monitored for metabolic acidosis and treated as in the nonpregnant state [see Warnings and Precautions (5.4)]. Newborns of mothers treated with topiramate should be monitored for metabolic acidosis because of transfer of topiramate to the fetus and possible occurrence of transient metabolic acidosis following birth.
There are no studies using topiramate in pregnant women. Topiramate should be used during pregnancy only if the potential benefit outweighs the potential risk to the fetus.
Topiramate has demonstrated selective developmental toxicity, including teratogenicity, in multiple animal species at clinically relevant doses. When oral doses of 20, 100 or 500 mg/kg were administered to pregnant mice during the period of organogenesis, the incidence of fetal malformations (primarily craniofacial defects) was increased at all doses. The low dose is approximately 0.2 times the recommended human dose (RHD) 400 mg/day on a mg/m2 basis. Fetal body weights and skeletal ossification were reduced at 500 mg/kg in conjunction with decreased maternal body weight gain.
In rat studies (oral doses of 20, 100, and 500 mg/kg or 0.2, 2.5, 30, and 400 mg/kg), the frequency of limb malformations (ectrodactyly, micromelia, and amelia) was increased among the offspring of dams treated with 400 mg/kg (10 times the RHD on a mg/m2 basis) or greater during the organogenesis period of pregnancy. Embryotoxicity (reduced fetal body weights, increased incidence of structural variations) was observed at doses as low as 20 mg/kg (0.5 times the RHD on a mg/m2 basis). Clinical signs of maternal toxicity were seen at 400 mg/kg and above, and maternal body weight gain was reduced during treatment with 100 mg/kg or greater.
In rabbit studies (20, 60, and 180 mg/kg or 10, 35, and 120 mg/kg orally during organogenesis), embryo/fetal mortality was increased at 35 mg/kg (2 times the RHD on a mg/m2 basis) or greater, and teratogenic effects (primarily rib and vertebral malformations) were observed at 120 mg/kg (6 times the RHD on a mg/m2 basis). Evidence of maternal toxicity (decreased body weight gain, clinical signs, and/or mortality) was seen at 35 mg/kg and above.
When female rats were treated during the latter part of gestation and throughout lactation (0.2, 4, 20, and 100 mg/kg or 2, 20, and 200 mg/kg), offspring exhibited decreased viability and delayed physical development at 200 mg/kg (5 times the RHD on a mg/m2 basis) and reductions in pre-and/or postweaning body weight gain at 2 mg/kg (0.05 times the RHD on a mg/m2 basis) and above. Maternal toxicity (decreased body weight gain, clinical signs) was evident at 100 mg/kg or greater.
In a rat embryo/fetal development study with a postnatal component (0.2, 2.5, 30 or 400 mg/kg during organogenesis; noted above), pups exhibited delayed physical development at 400 mg/kg (10 times the RHD on a mg/m2 basis) and persistent reductions in body weight gain at 30 mg/kg (1 times the RHD on a mg/m2 basis) and higher.
Pregnancy Registry
The North American Drug Pregnancy Registry has been established to collect information and provide scientific knowledge about safety and outcomes associated with pregnant women being treated with antiepileptic drugs. It is desirable that the experience from patients who are exposed to topiramate during pregnancy be reported to this registry. Such information can be reported to the North American Drug Pregnancy Registry by either a healthcare provider or the patient by calling 1-888-233-2334. Information about the North American Drug Pregnancy Registry can be found at http://www.massgeneral.org/aed/ .
8.2 Labor and Delivery
8.2 Labor and Delivery
Although the effect of topiramate on labor and delivery in humans has not been established, the development of topiramate-induced metabolic acidosis in the mother and/or in the fetus might affect the fetus’ ability to tolerate labor [see Pregnancy (8.1)].
8.3 Nursing Mothers
8.3 Nursing Mothers
Limited data on 5 breastfeeding infants exposed to topiramate showed infant plasma topiramate levels equal to 10-20% of the maternal plasma level. The effects of this exposure on infants are unknown. Caution should be exercised when administered to a nursing woman.
8.4 Pediatric Use
8.4 Pediatric Use
Adjunctive Treatment for Partial Onset Epilepsy in Infants and Toddlers (1 to 24 months)
Safety and effectiveness in patients below the age of 2 years have not been established for the adjunctive therapy treatment of partial onset seizures, primary generalized tonic-clonic seizures, or seizures associated with Lennox-Gastaut syndrome. In a single randomized, double-blind placebo-controlled investigational trial, the efficacy, safety, and tolerability of topiramate oral liquid and sprinkle formulations as an adjunct to concurrent antiepileptic drug therapy in infants 1 to 24 months of age with refractory partial onset seizures, was assessed. After 20 days of double-blind treatment, topiramate (at fixed doses of 5, 15, and 25 mg/kg per day) did not demonstrate efficacy compared with placebo in controlling seizures.
In general, the adverse reaction profile in this population was similar to that of older pediatric patients, although results from the above controlled study, and an open-label long-term extension study in these infants/toddlers (1 to 24 months old) suggested some adverse reactions/toxicities (not previously observed in older pediatric patients and adults; i.e, growth/length retardation, certain clinical laboratory abnormalities, and other adverse reactions/toxicities that occurred with a greater frequency and/or greater severity than had been recognized previously from studies in older pediatric patients or adults for various indications.
These very young pediatric patients appeared to experience an increased risk for infections (any topiramate dose 12%, placebo 0%) and of respiratory disorders (any topiramate dose 40%, placebo 16%). The following adverse reactions were observed in at least 3% of patients on topiramate and were 3% to 7% more frequent then in patients on placebo: viral infection, bronchitis, pharyngitis, rhinitis, otitis media, upper respiratory infection, cough, and bronchospasm. A generally similar profile was observed in older children [see Adverse Reactions (6)].
Topiramate resulted in an increased incidence of patients with increased creatinine (any topiramate dose 5%, placebo 0%), BUN (any topiramate dose 3%, placebo 0%), and protein (any topiramate dose 34%, placebo 6%), and an increased incidence of decreased potassium (any topiramate dose 7%, placebo 0%). This increased frequency of abnormal values was not dose-related. Creatinine was the only analyte showing a noteworthy increased incidence (topiramate 25 mg/kg/d 5%, placebo 0%) of a markedly abnormal increase [see Warnings and Precautions (5.13)]. The significance of these finding is uncertain.
Topiramate treatment also produced a dose-related increase in the percentage of patients who had a shift from normal at baseline to high/increased (above the normal reference range) in total eosinophil count at the end of treatment. The incidence of these abnormal shifts was 6 % for placebo, 10% for 5 mg/kg/d, 9% for 15 mg/kg/d, 14% for 25 mg/kg/d, and 11% for any topiramate dose [see Warnings and Precautions (5.13)]. There was a mean dose-related increase in alkaline phosphatase. The significance of these finding is uncertain.
Topiramate produced a dose-related increased incidence of treatment-emergent hyperammonemia. [see Warnings and Precautions (5.8)].
Treatment with topiramate for up to 1 year was associated with reductions in Z SCORES for length, weight, and head circumference. [see Warnings and Precautions (5.4) and Adverse Reactions (6)].
In open-label, uncontrolled experience, increasing impairment of adaptive behavior was documented in behavioral testing over time in this population. There was a suggestion that this effect was dose-related. However, because of the absence of an appropriate control group, it is not known if this decrement in function was treatment related or reflects the patient’s underlying disease (e.g., patients who received higher doses may have more severe underlying disease) [see Warnings and Precautions (5.5)].
In this open-label, uncontrolled study, the mortality was 37 deaths/1000 patient years. It is not possible to know whether this mortality rate is related to topiramate treatment, because the background mortality rate for a similar, significantly refractory, young pediatric population (1-24 months) with partial epilepsy is not known.
Monotherapy Treatment in Partial Onset Epilepsy in Patients less than 10 Years Old
Safety and effectiveness in patients below the age of 10 years have not been established for the monotherapy treatment of epilepsy.
Juvenile Animal Studies
When topiramate (30, 90 or 300 mg/kg/day) was administered orally to rats during the juvenile period of development (postnatal days 12 to 50), bone growth plate thickness was reduced in males at the highest dose, which is approximately 5-8 times the maximum recommended pediatric dose (9 mg/kg/day) on a body surface area (mg/m2) basis.
8.5 Geriatric Use 8.6 Race and Gender Effects 8.7 Renal Impairment 8.8 Patients Undergoing Hemodialysis
8.5 Geriatric Use
In clinical trials, 3% of patients were over 60. No age related difference in effectiveness or adverse effects were evident. However, clinical studies of topiramate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. Dosage adjustment may be necessary for elderly with impaired renal function (creatinine clearance rate less than 70 mL/min/1.73 m2) due to reduced clearance of topiramate [see Clinical Pharmacology (12.3) and Dosage and Administration (2.5)].
8.6 Race and Gender Effects
Evaluation of effectiveness and safety in clinical trials has shown no race or gender related effects.
8.7 Renal Impairment
The clearance of topiramate was reduced by 42% in moderately renally impaired (creatinine clearance 30 to 69 mL/min/1.73 m2) and by 54% in severely renally impaired subjects (creatinine clearance less than 30 mL/min/1.73 m2) compared to normal renal function subjects (creatinine clearance greater than 70 mL/min/1.73 m2). One-half the usual starting and maintenance dose is recommended in patients with moderate or severe renal impairment [see Dosage and Administration (2.6) and Clinical Pharmacology (12.4)].
8.8 Patients Undergoing Hemodialysis
Topiramate is cleared by hemodialysis at a rate that is 4 to 6 times greater than a normal individual. Accordingly, a prolonged period of dialysis may cause topiramate concentration to fall below that required to maintain an anti-seizure effect. To avoid rapid drops in topiramate plasma concentration during hemodialysis, a supplemental dose of topiramate may be required. The actual adjustment should take into account the duration of dialysis period, the clearance rate of the dialysis system being used, and the effective renal clearance of topiramate in the patient being dialyzed [see Dosage and Administration (2.4) and Clinical Pharmacology (12.4)].
9. DRUG ABUSE AND DEPENDENCE 9.1 Controlled Substance
9. DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Topiramate is not a controlled substance.
9.2 Abuse
9.2 Abuse
The abuse and dependence potential of topiramate has not been evaluated in human studies.
9.3 Dependence
9.3 Dependence
Topiramate has not been systematically studied in animals or humans for its potential for tolerance or physical dependence.
10 OVERDOSAGE
10. OVERDOSAGE
Overdoses of topiramate have been reported. Signs and symptoms included convulsions, drowsiness, speech disturbance, blurred vision, diplopia, mentation impaired, lethargy, abnormal coordination, stupor, hypotension, abdominal pain, agitation, dizziness and depression. The clinical consequences were not severe in most cases, but deaths have been reported after poly-drug overdoses involving topiramate.
Topiramate overdose has resulted in severe metabolic acidosis [see Warnings and Precautions (5.4)].
A patient who ingested a dose between 96 and 110 g topiramate was admitted to hospital with coma lasting 20 to 24 hours followed by full recovery after 3 to 4 days.
In acute topiramate overdose, if the ingestion is recent, the stomach should be emptied immediately by lavage or by induction of emesis. Activated charcoal has been shown to adsorb topiramate in vitro. Treatment should be appropriately supportive. Hemodialysis is an effective means of removing topiramate from the body.
11. DESCRIPTION
11. DESCRIPTION
Topiramate is a sulfamate-substituted monosaccharide. Topiramate tablets are available as 25 mg, 50 mg, 100 mg, and 200 mg round tablets for oral administration.
Topiramate, USP is a white crystalline powder with a bitter taste. Topiramate is most soluble in alkaline solutions containing sodium hydroxide or sodium phosphate and having a pH of 9 to 10. It is freely soluble in acetone, chloroform, dimethylsulfoxide, and ethanol. The solubility in water is 9.8 mg/mL. Its saturated solution has a pH of 6.3. Topiramate has the molecular formula C12H21NO8S and a molecular weight of 339.36. Topiramate is designated chemically as 2,3:4,5-Di-O-isopropylidene-β-D-fructopyranose sulfamate and has the following structural formula:
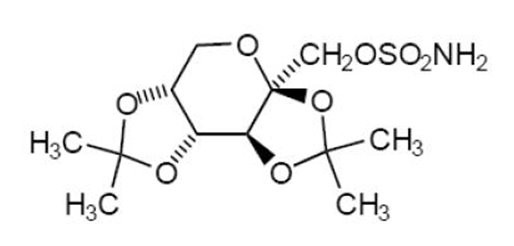
Topiramate tablets contain the following inactive ingredients: colloidal silicon dioxide, ferric oxide red (200 mg tablets), ferric oxide yellow (50, 100, and 200 mg tablets), hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, pregelatinized starch, sodium starch glycolate, talc and titanium dioxide.
12. CLINICAL PHARMACOLOGY 12.1 Mechanism of Action
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanisms by which topiramate exerts its anticonvulsant and other effects are unknown; however, preclinical studies have revealed four properties that may contribute to topiramate's efficacy for epilepsy and other indications. Electrophysiological and biochemical evidence suggests that topiramate, at pharmacologically relevant concentrations, blocks voltage-dependent sodium channels, augments the activity of the neurotransmitter gamma-aminobutyrate at some subtypes of the GABA-A receptor, antagonizes the AMPA/kainate subtype of the glutamate receptor, and inhibits the carbonic anhydrase enzyme, particularly isozymes II and IV.
12.2 Pharmacodynamics
12.2 Pharmacodynamics
Topiramate has anticonvulsant activity in rat and mouse maximal electroshock seizure (MES) tests. Topiramate is only weakly effective in blocking clonic seizures induced by the GABAA receptor antagonist, pentylenetetrazole. Topiramate is also effective in rodent models of epilepsy, which include tonic and absence-like seizures in the spontaneous epileptic rat (SER) and tonic and clonic seizures induced in rats by kindling of the amygdala or by global ischemia.
12.3 Pharmacokinetics 12.4 Special Populations 12.5 Drug-Drug Interactions
2.4 2.5 5.11
2.6
2.7
2.4 5.11
| AED Co-administered |
AED Concentration |
Topiramate Concentration |
| Phenytoin |
NC or 25% increasea |
48% decrease |
| Carbamazepine (CBZ) |
NC |
40% decrease |
| CBZ epoxideb |
NC |
NE |
| Valproic acid |
11% decrease |
14% decrease |
| Phenobarbital |
NC |
NE |
| Primidone |
NC |
NE |
| Lamotrigine |
NC at TPM doses up to 400 mg/day |
13% decrease |
5.8 7.1
7.2
7.3
7.4
7.5
7.5
Drug/Laboratory Tests Interactions
There are no known interactions of topiramate with commonly used laboratory tests.
13. NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
14. CLINICAL STUDIES 14.1 Monotherapy Epilepsy Controlled Trial
14. CLINICAL STUDIES
The studies described in the following sections were conducted using topiramate tablets.
14.1 Monotherapy Epilepsy Controlled Trial
The effectiveness of topiramate as initial monotherapy in adults and children 10 years of age and older with partial onset or primary generalized seizures was established in a multicenter, randomized, double-blind, parallel-group trial.
The trial was conducted in 487 patients diagnosed with epilepsy (6 to 83 years of age) who had 1 or 2 well-documented seizures during the 3-month retrospective baseline phase who then entered the study and received topiramate 25 mg/day for 7 days in an open-label fashion. Forty-nine percent of subjects had no prior AED treatment and 17% had a diagnosis of epilepsy for greater than 24 months. Any AED therapy used for temporary or emergency purposes was discontinued prior to randomization. In the double-blind phase, 470 patients were randomized to titrate up to 50 mg/day or 400 mg/day. If the target dose could not be achieved, patients were maintained on the maximum tolerated dose. Fifty eight percent of patients achieved the maximal dose of 400 mg/day for greater than 2 weeks, and patients who did not tolerate 150 mg/day were discontinued. The primary efficacy assessment was a between group comparison of time to first seizure during the double-blind phase. Comparison of the Kaplan-Meier survival curves of time to first seizure favored the topiramate 400 mg/day group over the topiramate 50 mg/day group (p=0.0002, log rank test; Figure 1). The treatment effects with respect to time to first seizure were consistent across various patient subgroups defined by age, sex, geographic region, baseline body weight, baseline seizure type, time since diagnosis, and baseline AED use.
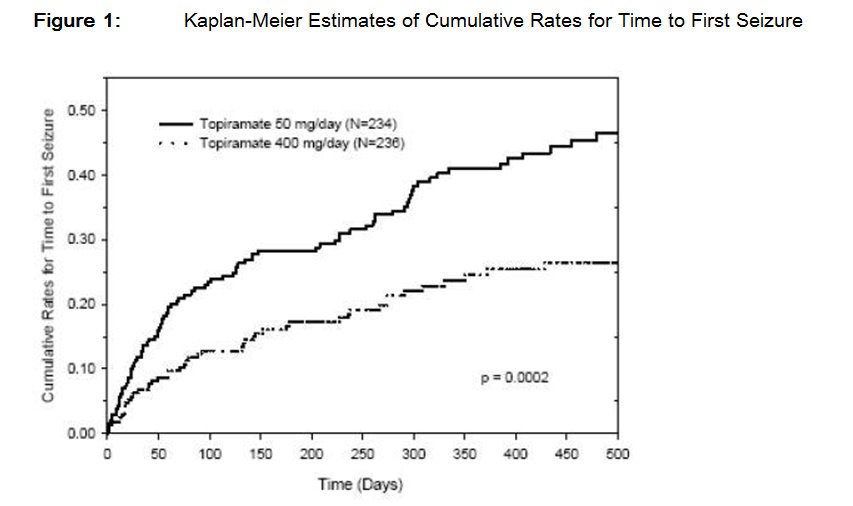
|
|
|
|
Target Topiramate Dosage (mg/day) |
|
|
|
|
| Protocol | Stabilization Dose | Placeboa |
200 |
400 |
600 |
900 |
1000 |
| YD |
N |
42 |
42 |
40 |
41 |
- - |
- - |
|
|
Mean Dose |
5.9 |
200 |
390 |
556 |
- - |
- - |
|
|
Median Dose |
6.0 |
200 |
400 |
600 |
- - |
- - |
| YE |
N |
44 |
- - |
- - |
40 |
45 |
40 |
|
|
Mean Dose |
9.7 |
- - |
- - |
544 |
739 |
796 |
|
|
Median Dose |
10.0 |
- - |
- - |
600 |
800 |
1000 |
| Y1 |
N |
23 |
- - |
19 |
- - |
- - |
- - |
|
|
Mean Dose |
3.8 |
- - |
395 |
- - |
- - |
- - |
|
|
Median Dose |
4.0 |
- - |
400 |
- - |
- - |
- - |
| Y2 |
N |
30 |
- - |
- - |
28 |
- - |
- - |
|
|
Mean Dose |
5.7 |
- - |
- - |
522 |
- - |
- - |
|
|
Median Dose |
6.0 |
- - |
- - |
600 |
- - |
- - |
| Y3 |
N |
28 |
- - |
- - |
- - |
25 |
- - |
|
|
Mean Dose |
7.9 |
- - |
- - |
- - |
568 |
- - |
|
|
Median Dose |
8.0 |
- - |
- - |
- - |
600 |
- - |
| 119 |
N |
90 |
157 |
- - |
- - |
- - |
- - |
|
|
Mean Dose |
8 |
200 |
- - |
- - |
- - |
- - |
|
|
Median Dose |
8 |
200 |
- - |
- - |
- - |
- - |
|
|
|
|
Target Topiramate Dosage (mg/day) |
|
|
|
|
|
| Protocol Efficacy Results |
|
Placebo |
200 |
400 |
600 |
800 |
1000 |
≈6 mg/kg/day* |
| Partial Onset Seizures Studies in Adults |
|
|
|
|
|
|
|
|
| YD |
N |
45 |
45 |
45 |
46 |
- - |
- - |
- - |
|
|
Median % Reduction |
11.6 |
27.2a |
47.5b |
44.7c |
- - | - - | - - |
|
|
% Responders |
18 |
24 |
44d |
46d |
- - | - - | - - |
| YE |
N |
47 |
- - |
- - |
48 |
48 |
47 |
- - |
|
|
Median % Reduction |
1.7 |
- - |
- - |
40.8c |
41.0c |
36.0c |
- - |
|
|
% Responders |
9 |
- - | - - | 40 c |
41 c |
36d |
- - |
| Y1 |
N |
24 |
- - |
23 |
- - |
- - |
- - |
- - |
|
|
Median % Reduction |
1.1 |
- - |
40.7e |
- - |
- - |
- - |
- - |
|
|
% Responders |
8 |
- - |
35d |
- - |
- - |
- - |
- - |
| Y2 |
N |
30 |
- - |
- - |
30 |
- - |
- - |
- - |
|
|
Median % Reduction |
-12.2 |
- - |
- - |
46.4f |
- - |
- - |
- - |
|
|
% Responders |
10 |
- - |
- - |
47c |
- - |
- - |
- - |
| Y3 |
N |
28 |
- - |
- - |
- - |
28 |
- - |
- - |
|
|
Median % Reduction |
-20.6 |
- - |
- - |
- - |
24.3c |
- - |
- - |
|
|
% Responders |
0 |
- - |
- - |
- - |
43c |
- - |
- - |
| 119 |
N |
91 |
168 |
- - |
- - |
- - |
- - |
- - |
|
|
Median % Reduction |
20.0 |
44.2c |
- - |
- - |
- - |
- - |
- - |
|
|
% Responders |
24 |
45c |
- - |
- - |
- - |
- - |
- - |
| Studies in Pediatric Patients |
|
|
|
|
|
|
|
|
| YP |
N |
45 |
- - |
- - |
- - |
- - |
- - |
41 |
|
|
Median % Reduction |
10.5 |
- - |
- - |
- - |
- - |
- - |
33.1d |
|
|
% Responders |
20 |
- - |
- - |
- - |
- - |
- - |
39 |
| Primary Generalized Tonic-Clonich |
|
|
|
|
|
|
|
|
| YTC |
N |
40 |
- - |
- - |
- - |
- - |
- - |
39 |
|
|
Median % Reduction |
9.0 |
- - |
- - |
- - |
- - |
- - |
56.7 d |
|
|
% Responders |
20 |
- - |
- - |
- - |
- - |
- - |
56c |
| Lennox-Gastaut Syndromei |
|
|
|
|
|
|
|
|
| YL |
N |
49 |
- - |
- - |
- - |
- - |
- - |
46 |
|
|
Median % Reduction |
-5.1 |
- - |
- - |
- - |
- - |
- - |
14.8d |
|
|
% Responders |
14 |
- - |
- - |
- - |
- - |
- - |
28g |
| Improvement in Seizure severityj |
28 |
- - |
- - |
- - |
- - |
- - |
- - |
52d |
16. HOW SUPPLIED/STORAGE AND HANDLING Topiramate tablets
16. HOW SUPPLIED/STORAGE AND HANDLING
Topiramate Tablets 25 mg are white to off white, round, biconvex, film coated tablets debossed with ‘1031’ on one side and ‘25’ on other side. Topiramate Tablets 25 mg are supplied as follows:
| Package |
NDC Number |
| Bottles of 30 |
NDC 13668-031-30 |
| Bottles of 60 |
NDC 13668-031-60 |
| Bottles of 100 |
NDC 13668-031-01 |
| Bottles of 500 | NDC 13668-031-05 |
| Bottles of 9000 |
NDC 13668-031-53 |
| 100 unit dose tablets |
NDC 13668-031-74 |
| Package |
NDC Number |
| Bottles of 30 |
NDC 13668-032-30 |
| Bottles of 60 |
NDC 13668-032-60 |
| Bottles of 100 |
NDC 13668-032-01 |
| Bottles of 500 |
NDC 13668-032-05 |
| Bottles of 6000 |
NDC 13668-032-42 |
| 100 unit dose tablets |
NDC 13668-032-74 |
| Package |
NDC Number |
| Bottles of 60 |
NDC 13668-033-60 |
| Bottles of 100 |
NDC 13668-033-01 |
| Bottles of 500 |
NDC 13668-033-05 |
| Bottles of 2500 |
NDC 13668-033-31 |
| 90 unit dose tablets |
NDC 13668-033-64 |
| Package |
NDC Number |
| Bottles of 60 |
NDC 13668-034-60 |
| Bottles of 100 |
NDC 13668-034-01 |
| Bottles of 500 |
NDC 13668-034-05 |
| Bottles of 1500 |
NDC 13668-034-15 |
| 80 unit dose tablets |
NDC 13668-034-77 |
Storage and Handling
17. PATIENT COUNSELING INFORMATION 17.1 Eye Disorders 17.2 Oligohydrosis and Hyperthermia 17.3 Suicidal Behavior and Ideation 17.4 Metabolic Acidosis 17.5 Interference with Cognitive and Motor Performance 17.6 Hyperammonemia and Encephalopathy 17.7 Kidney
FDA approved Medication Guide
5.1
5.2
5.4
5.5
5.8
5.9
8.18.38.1
500 Tablets NDC 13668-033-05
Topiramate Tablets
100 mg
PHARMACIST: PLEASE DISPENSE WITH MEDICATION GUIDE PROVIDED SEPARATELY.
torrent Rx only
Manufactured by:
TORRENT PHARMACEUTICALS LTD.,
Indrad-382 721
Dist. Mehsana, INDIA.
For:
TORRENT PHARMA INC.,
5380 Holiday Terrace, Suite 40,
Kalamazoo, Michigan 49009.
Each tablet contains 100 mg of topiramate, USP,
Store at 20° - 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F) [see USP Controlled Room Temperature].
PROTECT FROM MOISTURE
Dispense in a tight, light-resistant container with child resistant closure.
Usual Dosage:
See accompanying prescribing information.
Mgd. Lic. No. : G/926
N3 13668-033-05 2
Batch
EXP
8012527
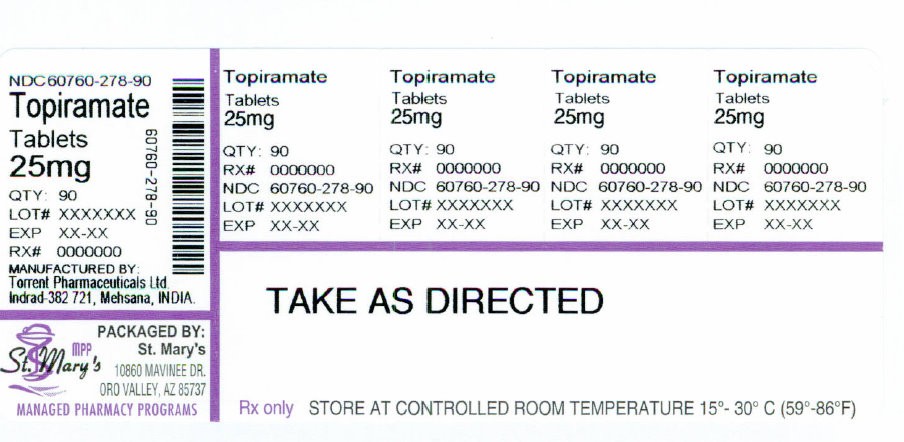

TOPIRAMATETOPIRAMATE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TOPIRAMATETOPIRAMATE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TOPIRAMATETOPIRAMATE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||