Topotecan Hydrochloride
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Topotecan Hydrochloride for Injection safely and effectively. See full prescribing information for Topotecan Hydrochloride for Injection.Topotecan Hydrochloride for InjectionInitial U.S. Approval: 1996 BOXED WARNINGWARNING: BONE MARROW SUPPRESSION See full prescribing information for complete boxed warning. 35.1INDICATIONS AND USAGETopotecan Hydrochloride for Injection is a topoisomerase inhibitor indicated for: small cell lung cancer sensitive disease after failure of first-line chemotherapy. (1) combination therapy with cisplatin for stage IV-B, recurrent, or persistent carcinoma of the cervix which is not amenable to curative treatment with surgery and/or radiation therapy. (1) DOSAGE AND ADMINISTRATION Small cell lung cancer: 1.5 mg/m2 by intravenous infusion over 30 minutes daily for 5 consecutive days, starting on day one of a 21-day course. (2.1) Cervical cancer: 0.75 mg/m2 by intravenous infusion over 30 minutes on days 1, 2, and 3 followed by cisplatin 50 mg/m2 by intravenous infusion on day 1 repeated every 21 days. (2.2) See Dosage Modification Guidelines for patients with neutropenia or reduced platelets. (2.1, 2.2)See Dosage Adjustment in Renal Impairment. (2.3)DOSAGE FORMS AND STRENGTHS3CONTRAINDICATIONS History of severe hypersensitivity reactions (e.g., anaphylactoid reactions) to topotecan or any of its ingredients (4) Severe bone marrow depression (4) WARNINGS AND PRECAUTIONS Bone marrow suppression: Administer topotecan hydrochloride only to patients with adequate bone marrow reserves. Monitor peripheral blood counts and adjust the dose if needed. (5.1) Topotecan-induced neutropenia can lead to neutropenic colitis. (5.2) Interstitial lung disease: Topotecan hydrochloride has been associated with reports of interstitial lung disease. Monitor patients for symptoms and discontinue topotecan hydrochloride if the diagnosis is confirmed. (5.3) Pregnancy: Can cause fetal harm. Advise women of potential risk to the fetus. (5.4, 8.1) Side EffectsSmall cell lung cancer: The most common hematologic adverse reactions were: neutropenia (97%), leukopenia (97%), anemia (89%), and thrombocytopenia (69%). (6.1) The most common (>25%) non-hematologic adverse reactions (all grades) were: nausea, alopecia, vomiting, sepsis or pyrexia/infection with neutropenia, diarrhea, constipation, fatigue, and pyrexia. (6.1) Cervical cancer (Topotecan hydrochloride plus cisplatin): The most common hematologic adverse reactions (all grades) were: anemia (94%), leukopenia (91%), neutropenia (89%), and thrombocytopenia (74%). (6.1) The most common (>25%) non-hematologic adverse reactions (all grades) were: pain, nausea, vomiting, and infection/febrile neutropenia. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact APP Pharmaceuticals, LLC, Medical Affairs at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Do not initiate G-CSF until 24 hours after completion of treatment with topotecan hydrochloride. Concomitant administration can prolong duration of neutropenia. (7) Greater myelosuppression is likely to be seen when used in combination with other cytotoxic agents. (7) USE IN SPECIFIC POPULATIONS Nursing Mothers: Discontinue nursing when receiving topotecan hydrochloride. (8.3)
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: BONE MARROW SUPPRESSION
- 1 INDICATIONS & USAGE
- 2 DOSAGE & ADMINISTRATION
- 3 DOSAGE FORMS & STRENGTHS
- 4 TOPOTECAN HYDROCHLORIDE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 TOPOTECAN HYDROCHLORIDE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 TOPOTECAN HYDROCHLORIDE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY – Topotecan Hydrochloride for Injection 4 mg Single Dose Vial
FULL PRESCRIBING INFORMATION
WARNING: BONE MARROW SUPPRESSION
Do not give topotecan hydrochloride to patients with baseline neutrophil counts less than 1,500 cells/mm3. In order to monitor the occurrence of bone marrow suppression, primarily neutropenia, which may be severe and result in infection and death, monitor peripheral blood counts frequently on all patients receiving topotecan hydrochloride [see Warnings and Precautions (5.1)].
1 INDICATIONS & USAGE
Topotecan hydrochloride is indicated for the treatment of:
- small cell lung cancer sensitive disease after failure of first-line chemotherapy. In clinical studies submitted to support approval, sensitive disease was defined as disease responding to chemotherapy but subsequently progressing at least 60 days (in the Phase 3 study) or at least 90 days (in the Phase 2 studies) after chemotherapy [see Clinical Studies (14)].
Topotecan hydrochloride in combination with cisplatin is indicated for the treatment of:
- stage IV-B, recurrent, or persistent carcinoma of the cervix which is not amenable to curative treatment with surgery and/or radiation therapy.
2 DOSAGE & ADMINISTRATION
33
2.1 Small Cell Lung Cancer
Recommended Dosage
- The recommended dose of topotecan hydrochloride is 1.5 mg/m2 by intravenous infusion over 30 minutes daily for 5 consecutive days, starting on day 1 of a 21-day course.
- In the absence of tumor progression, a minimum of 4 courses is recommended because tumor response may be delayed. The median time to response in 4 small cell lung cancer trials was 5 to 7 weeks.
Dosage Modification Guidelines
- In the event of severe neutropenia (defined as <500 cells/mm3) during any course, reduce the dose by 0.25 mg/m2 (to 1.25 mg/m2) for subsequent courses.
- Alternatively, in the event of severe neutropenia, administer G-CSF (granulocyte-colony stimulating factor) following the subsequent course (before resorting to dose reduction) starting from day 6 of the course (24 hours after completion of topotecan administration).
- In the event the platelet count falls below 25,000 cells/mm3, reduce doses by 0.25 mg/m2 (to 1.25 mg/m2) for subsequent courses.
2.2 Cervical Cancer
Recommended Dosage
The recommended dose of topotecan hydrochloride is 0.75 mg/m2 by intravenous infusion over 30 minutes daily on days 1, 2, and 3; followed by cisplatin 50 mg/m2 by intravenous infusion on day 1 repeated every 21 days (a 21-day course).
Dosage Modification Guidelines
Dosage adjustments for subsequent courses of topotecan hydrochloride in combination with cisplatin are specific for each drug. See manufacturer’s prescribing information for cisplatin administration and hydration guidelines and for cisplatin dosage adjustment in the event of hematologic toxicity.
- In the event of severe febrile neutropenia (defined as <1,000 cells/mm3 with temperature of 38°C or 100.4°F), reduce the dose of topotecan hydrochloride to 0.6 mg/m2 for subsequent courses.
- Alternatively, in the event of severe febrile neutropenia, administer G-CSF following the subsequent course (before resorting to dose reduction) starting from day 4 of the course (24 hours after completion of administration of topotecan hydrochloride).
- If febrile neutropenia occurs despite the use of G-CSF, reduce the dose of topotecan hydrochloride to 0.45 mg/m2 for subsequent courses.
- In the event the platelet count falls below 25,000 cells/mm3, reduce doses to 0.6 mg/m2 for subsequent courses.
2.3 Dosage Adjustment in Specific Populations
Renal Impairment
No dosage adjustment of topotecan hydrochloride appears to be required for patients with mild renal impairment (Clcr 40 to 60 mL/min.). Dosage adjustment of topotecan hydrochloride to 0.75 mg/m2 is recommended for patients with moderate renal impairment (20 to 39 mL/min.). Insufficient data are available in patients with severe renal impairment to provide a dosage recommendation for topotecan hydrochloride [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Topotecan hydrochloride in combination with cisplatin for the treatment of cervical cancer should only be initiated in patients with serum creatinine ≤1.5 mg/dL. In the clinical trial, cisplatin was discontinued for a serum creatinine >1.5 mg/dL. Insufficient data are available regarding continuing monotherapy with topotecan hydrochloride after cisplatin discontinuation in patients with cervical cancer.
2.4 Instructions for Handling, Preparation and Intravenous Administration
Handling
Topotecan hydrochloride is a cytotoxic anticancer drug. Prepare topotecan hydrochloride under a vertical laminar flow hood while wearing gloves and protective clothing. If topotecan hydrochloride solution contacts the skin, wash the skin immediately and thoroughly with soap and water. If topotecan hydrochloride contacts mucous membranes, flush thoroughly with water.
Use procedures for proper handling and disposal of anticancer drugs. Several guidelines on this subject have been published.1-4
Preparation and Administration
Each 4 mg vial of topotecan hydrochloride is reconstituted with 4 mL Sterile Water for Injection. Then the appropriate volume of the reconstituted solution is diluted in either 0.9% Sodium Chloride Intravenous Infusion or 5% Dextrose Intravenous Infusion prior to administration.
Stability
Unopened vials of topotecan hydrochloride are stable until the date indicated on the package when stored between 20° and 25°C (68° and 77°F) [see USP Controlled Room Temperature] and protected from light in the original package. Because the vials contain no preservative, contents should be used immediately after reconstitution.
Reconstituted vials of topotecan hydrochloride diluted for infusion are stable at approximately 20° to 25°C (68° to 77°F) and ambient lighting conditions for 24 hours.
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Bone Marrow Suppression
Bone marrow suppression (primarily neutropenia) is the dose-limiting toxicity of topotecan hydrochloride. Neutropenia is not cumulative over time. In the comparative study, in small cell lung cancer, the treatment-related death rates were 5% for topotecan hydrochloride and 4% for CAV (cyclophosphamide-doxorubicin-vincristine).
Neutropenia
- Small cell lung cancer experience: Grade 4 neutropenia (<500 cells/mm3) was most common during course 1 of treatment (60% of patients) and occurred in 39% of all courses, with a median duration of 7 days. The nadir neutrophil count occurred at a median of 12 days. Therapy-related sepsis or febrile neutropenia occurred in 23% of patients, and sepsis was fatal in 1%. Pancytopenia has been reported.
- Cervical cancer experience: Grade 3 and grade 4 neutropenia affected 26% and 48% of patients, respectively.
Thrombocytopenia
- Small cell lung cancer experience: Grade 4 thrombocytopenia (<25,000/mm3) occurred in 27% of patients and in 9% of courses, with a median duration of 5 days and platelet nadir at a median of 15 days. Platelet transfusions were given to 15% of patients in 4% of courses.
- Cervical cancer experience: Grade 3 and grade 4 thrombocytopenia affected 26% and 7% of patients, respectively.
Anemia
- Small cell lung cancer experience: Grade 3/4 anemia (<8 g/dL) occurred in 37% of patients and in 14% of courses. Median nadir was at day 15. Transfusions were needed in 52% of patients in 22% of courses.
- Cervical cancer experience: Grade 3 and grade 4 anemia affected 34% and 6% of patients, respectively.
Monitoring of Bone Marrow Function
3333[see Drug Interactions (7)].5.2 Neutropenic Colitis
5.3 Interstitial Lung Disease
[see Adverse Reactions (6.2)].
5.4 Pregnancy
Pregnancy Category D
Topotecan hydrochloride can cause fetal harm when administered to a pregnant woman.
Topotecan caused embryolethality, fetotoxicity, and teratogenicity in rats and rabbits when administered during organogenesis. There are no adequate and well controlled studies of topotecan hydrochloride in pregnant women. If this drug is used during pregnancy, or if a patient becomes pregnant while receiving topotecan hydrochloride, the patient should be apprised of the potential hazard to the fetus [see Use in Specific Populations, Pregnancy (8.1)].
5.5 Inadvertent Extravasation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Small Cell Lung Cancer
Data in the following section are based on the combined experiences of the 879 patients studied, including 426 patients with small cell lung cancer treated with topotecan hydrochloride injection. Table 1 lists the principal hematologic adverse reactions, and Table 2 lists non-hematologic adverse reactions occurring in at least 15% of patients.
Table 1. Hematologic Adverse Reactions Experienced in ≥ 15% of Patients Receiving Topotecan Hydrochloride
| Hematologic Adverse Reaction |
Patients (n=879) % Incidence |
|---|---|
| Neutropenia | |
| < 1,500 cells/mm3 | 97 |
| < 500 cells/mm3 | 78 |
| Leukopenia | |
| < 3,000 cells/mm3 | 97 |
| < 1,000 cells/mm3 | 32 |
| Thrombocytopenia | |
| < 75,000 cells/mm3 | 69 |
| < 25,000 cellls/mm3 | 27 |
| Anemia | |
| < 10 g/dL | 89 |
| < 8 g/dL | 37 |
Table 2. Non-hematologic Adverse Reactions Experienced by ≥ 15% of Patients Receiving Topotecan Hydrochloride
| Non-hematologic Adverse Reaction |
Percentage of Patients with Adverse Reaction (879 Patients) | ||
|---|---|---|---|
| All Grades | Grade 3 | Grade 4 | |
|
NA=Not applicable NR=Not reported separately a Does not include Grade 1 sepsis or pyrexia. b Rash also includes pruritus, rash erythematous, urticaria, dermatitis, bullous eruption, and maculopapular rash. c Pain includes body pain, back pain, and skeletal pain. |
|||
| Infections and infestations Sepsis or pyrexia/infection with neutropeniaa |
43 |
NR |
23 |
| Metabolism and nutrition disorders Anorexia |
19 |
2 |
< 1 |
| Nervous system disorders Headache |
18 |
1 |
< 1 |
| Respiratory, thoracic, and mediastinal disorders
Dyspnea Coughing |
22 15 |
5 1 |
3 0 |
| Gastrointestinal disorders Nausea Vometing Diarrhea Constipation Abdominal pain Stomatitis |
64 45 32 29 22 18 |
7 4 3 2 2 1 |
1 1 1 1 2 < 1 |
|
Skin and subcutaneous tissue disorders Alopecia Rashb |
49 16 |
NA 1 |
NA 0 |
|
General disorders and administrative disorders site conditions
Fatigue Pyrexia Painc Asthenia |
29 28 23 25 |
5 1 2 4 |
0 < 1 1 2 |
Nervous System Disorders
Paresthesia occurred in 7% of patients but was generally grade 1.
Hepatobiliary Disorders
Grade 1 transient elevations in hepatic enzymes occurred in 8% of patients. Greater elevations, grade 3/4, occurred in 4%. Grade 3/4 elevated bilirubin occurred in <2% of patients.
Table 4 shows the grade 3/4 hematologic and major non-hematologic adverse reactions in the topotecan/CAV comparator trial in small cell lung cancer.
Table 4. Adverse Reactions Experienced by ≥ 5% of Small Cell Lung Cancer Patients Randomized to Receive Topotecan Hydrochloride or CAV
| Adverse Reaction | Topotecan Hydrochloride (n=107) |
CAV (n=104) |
|---|---|---|
|
a Death related to sepsis occurred in 3% of patients receiving topotecan hydrochloride, and 1% of patients receiving CAV. b Pain includes body pain, skeletal pain, and back pain. |
||
| Hematologic Grade 3/4 |
%
|
%
|
| Grade 4 neutropenia (<500 cells/mm3) |
70 |
72 |
| Grade 3/4 anemia (Hgb < 8 g/dL) |
42 |
20 |
| Grade 4 thrombocytopenia (<25,000 plts/mm3) |
29 |
5 |
| Pyrexia/Grade 4 neutropenia | 28 |
26 |
| Non-hematologic Grade 3/4 |
%
|
%
|
|
Infections and infestations
Documented sepsisa |
5 |
5 |
|
Respiratory, thoracic, and mediastinal disorders Dyspnea Pneumonia |
9 8 |
14 6 |
|
Gastrointestinal disorders
Abdominal pain Nausea |
6 8 |
4 6 |
|
General disorders and administrative site
conditions
Fatigue Asthenia Painb |
6 9 5 |
10 7 7 |
Cervical Cancer
In the comparative trial with topotecan hydrochloride plus cisplatin versus cisplatin in patients with cervical cancer, the most common dose-limiting adverse reaction was myelosuppression. Table 5 shows the hematologic adverse reactions and Table 6 shows the non-hematologic adverse reactions in patients with cervical cancer.
Table 5. Hematologic Adverse Reactions in Patients with Cervical Cancer Treated with Topotecan Hydrochloride Plus Cisplatin or Cisplatin Monotherapya
| Hematologic Adverse Reaction |
Topotecan Hydrochloride Plus Cisplatin (n = 140) |
Cisplatin (n = 144) |
|---|---|---|
|
a Includes patients who were eligible and treated. |
||
| Anemia All grades (Hgb < 12 g/dL) Grade 3 (Hgb <8 to 6.5 g/dL) Grade 4 (Hgb < 6.5 g/dL) |
131 (94%) 47 (34%) 9 (6%) |
130 (90%) 28 (19 %) 5 (3%) |
| Leukopenia All Grades (< 3,800 cells/mm3) Grade 3 (< 2,000 to 1,000 cells/mm3) Grade 4 (< 1,000 cells/mm3) |
128 (91%) 58 (41%) 35 (25 %) |
43 (30%) 1 (1%) 0 (0%) |
| Neutropenia All Grades (< 2,000 Cells/mm3) Grade 3 (<1, 000 to 500 cells/mm3) Grade 4 (< 500 cells/mm3) |
125 (89 %) 36 (26 %) 67 (48%) |
28 (19%) 1 (1%) 1 (1%) |
| Thrombocytopenia All Grades (< 130,000 cells/mm3) Grade 3 (<50,000 to 10,000 cells/mm3) Grade 4 (< 10,000 cells/mm3) |
104 (74%) 36 (26%) 10 (7%) |
21 (15%) 5 (3%) 0 (0 %) |
Table 6. Non-hematologic Adverse Reactions Experienced by ≥5% of Patients with Cervical Cancer Treated with Topotecan Hydrochloride Plus Cisplatin or Cisplatin Monotherapya
| Adverse Reaction | Topotecan Hydrochloride Plus Cisplatin (n=140) |
Cisplatin (n=144) |
||||
|---|---|---|---|---|---|---|
| All Gradesb | Grade 3 | Grade 4 | All Gradesb | Grade 3 | Grade 4 | |
|
General disorders and administrative site conditions Constitutionalc Paind |
96 (69 %) 82 (59%) |
11 (8%) 28 (20%) |
0 3 (2%) |
89 (62%) 72 (50%) |
17 (12%) 18 (13%) |
0 5 (3%) |
| Gastrointestinal disorders Vomiting Nausea Stomatitis-pharyngitis Other |
56 (40%) 77 (55%) 8 (6%) 88 (63%) |
20 (14%) 18 (13%) 1 (< 1%) 16 (11%) |
2 (1%) 2 (1%) 0 4 (3 %) |
53 (37%) 79 (55%) 0 80 (56%) |
13 (9%) 13 (9%) 0 12 (8%) |
0 0 0 3 (2%) |
| Dermatology | 67 (48%) |
1 (<1%) |
0 |
29 (20%) |
0 |
0 |
| Metobolic-Laboratory | 55 (39%) |
13 (9%) |
7 (5%) |
44 (31%) |
14 (10%) |
1 (<1%) |
| Genitourinary | 51 (36%) |
9 (6%) |
9 (6%) |
49 (34%) |
7 (5%) |
7 (5%) |
|
Nervous system disorders
Neuropathy Other |
4 (3%) 49 (35%) |
1 (<1%) 3 (2%) |
0 1 (<1%) |
3 (2%) 43 (30%) |
1 (<1%) 7 (5%) |
0 2 (1%) |
| Infection-febrile neutopenia | 39 (28%) |
21 (15%) |
5 (4%) |
26 (18%) |
11 (8%) |
0 |
| Cardiovascular | 35 (25%) |
7 (5%) |
6 (4%) |
22 (15%) |
8 (6%) |
3 (2%) |
| Hepatic | 34 (24%) |
5 (4%) |
2 (1%) |
23 (16%) |
2 (1%) |
0 |
| Pulmonary | 24 (17%) |
4 (3%) |
0 |
23 (16%) |
5 (3%) |
3 (2%) |
|
Vascular disorders Hemorrhage Coagulation |
21 (15%) 8 (6%) |
8 (6%) 4 (3%) |
1 (<1%) 3 (2%) |
20 (14%) 10 (7%) |
3 (2%) 7 (5%) |
1 (<1%) 0 |
| Musculoskeletal | 19 (14%) |
3 (2%) |
0 |
7 (5%) |
1 (<1%) |
1 (<1%) |
| Allergy-Immunology | 8 (6%) |
2 (1%) |
1 (<1%) |
4 (3%) |
0 |
1 (<1%) |
| Endocrine | 8 (6%) |
0 |
1 (<1%) |
4 (3%) |
2 (1%) |
0 |
| Sexual reproduction function | 7 (5%) |
0 |
0 |
10 (7%) |
1 (<1%) |
0 |
| Ocular-visual | 7 (5%) |
0 |
0 |
7 (5%) |
1 (<1%) |
0 |
a
b
c
d
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials or listed in other sections of the prescribing information, the following reactions have been identified during post-marketing use of topotecan hydrochloride. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential casual connection to topotecan hydrochloride.
Blood and Lymphatic System Disorders: Severe bleeding (in association with thrombocytopenia) [see Warnings and Precautions (5.1)].
Immune System Disorders: Allergic manifestations; Anaphylactoid reactions.
Gastrointestinal Disorders: Abdominal pain potentially associated with neutropenic colitis [see Warnings and Precautions (5.2)].
Pulmonary Disorders: Interstitial lung disease [see Warnings and Precautions (5.3)].
Skin and Subcutaneous Tissue Disorders: Angioedema, severe dermatitis, severe pruritus.
General Disorders and Administration Site Conditions:[see Warnings and Precautions (5.5)].7 DRUG INTERACTIONS
G-CSF
Concomitant administration of G-CSF can prolong the duration of neutropenia, so if G-CSF is to be used, do not initiate it until day 6 of the course of therapy, 24 hours after completion of treatment with topotecan hydrochloride.
Platinum and Other Cytotoxic Agents
Myelosuppression was more severe when topotecan hydrochloride, at a dose of 1.25 mg/m2/day for 5 days, was given in combination with cisplatin at a dose of 50 mg/m2 in Phase 1 studies. In one study, 1 of 3 patients had severe neutropenia for 12 days and a second patient died with neutropenic sepsis.
Greater myelosuppression is also likely to be seen when topotecan hydrochloride is used in combination with other cytotoxic agents, thereby necessitating a dose reduction. However, when combining topotecan hydrochloride with platinum agents (e.g., cisplatin or carboplatin), a distinct sequence-dependent interaction on myelosuppression has been reported. Co-administration of a platinum agent on day 1 of dosing with topotecan hydrochloride required lower doses of each agent compared to co-administration on day 5 of the dosing schedule for topotecan hydrochloride.
22see Dosage and Administration (2), Adverse Reactions (6), Clinical Pharmacology (12.3), and Clinical Studies (14)8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D [see Warnings and Precautions (5.4) ].
Topotecan hydrochloride can cause fetal harm when administered to a pregnant woman. In rabbits, a dose of 0.1 mg/kg/day (about equal to the clinical dose on a mg/m2 basis) given on days 6 through 20 of gestation caused maternal toxicity, embryolethality, and reduced fetal body weight. In the rat, a dose of 0.23 mg/kg/day (about equal to the clinical dose on a mg/m2 basis) given for 14 days before mating through gestation day 6 caused fetal resorption, microphthalmia, pre-implant loss, and mild maternal toxicity. A dose of 0.1 mg/kg/day (about half the clinical dose on a mg/m2 basis) given to rats on days 6 through 17 of gestation caused an increase in post-implantation mortality. This dose also caused an increase in total fetal malformations. The most frequent malformations were of the eye (microphthalmia, anophthalmia, rosette formation of the retina, coloboma of the retina, ectopic orbit), brain (dilated lateral and third ventricles), skull, and vertebrae.
see Warnings and Precautions (5.4)8.3 Nursing Mothers
22
8.4 Pediatric Use
8.5 Geriatric Use
Of the 879 patients with cancer in clinical studies of topotecan hydrochloride, 32% (n=281) were 65 years of age and older, while 3.8% (n=33) were 75 years of age and older. Of the 140 patients with stage IV-B, relapsed, or refractory cervical cancer in clinical studies of topotecan hydrochloride who received topotecan hydrochloride plus cisplatin in the randomized clinical trial, 6% (n = 9) were 65 years of age and older, while 3% (n = 4) were 75 years of age and older. No overall differences in effectiveness or safety were observed between these patients and younger adult patients, and other reported clinical experience has not identified differences in responses between the elderly and younger adult patients, but greater sensitivity of some older individuals cannot be ruled out.
[see Clinical Pharmacology (12.3)].
[see Dosage and Administration (2.3)].
8.6 Renal Impairment
No dosage adjustment of topotecan hydrochloride appears to be required for patients with mild renal impairment (Clcr 40 to 60 mL/min.). Dosage reduction is recommended for patients with moderate renal impairment (Clcr 20 to 39 mL/min.). Insufficient data are available in patients with severe renal impairment to provide a dosage recommendation for topotecan hydrochloride [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
There is no known antidote for overdosage with topotecan hydrochloride. The primary anticipated complication of overdosage would consist of bone marrow suppression.
One patient on a single-dose regimen of 17.5 mg/m2 given on day 1 of a 21-day cycle had received a single dose of 35 mg/m2. This patient experienced severe neutropenia (nadir of 320/mm3) 14 days later but recovered without incident.
11 DESCRIPTION
Topotecan Hydrochloride for Injection is a semi-synthetic derivative of camptothecin and is an anti-tumor drug with topoisomerase I-inhibitory activity.
Topotecan Hydrochloride for Injection is supplied as a sterile lyophilized, buffered, light yellow to greenish powder available in single-dose vials. Each vial contains topotecan hydrochloride equivalent to 4 mg of topotecan as free base. The reconstituted solution ranges in color from yellow to yellow-green and is intended for administration by intravenous infusion.
Inactive ingredients are mannitol, 48 mg, and tartaric acid, 20 mg. Hydrochloric acid and sodium hydroxide may be used to adjust the pH. The solution pH ranges from 2.5 to 3.5.
The chemical name for topotecan hydrochloride is (S)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3’,4’:6,7] indolizino [1,2-b]quinoline-3,14-(4H,12H)-dione monohydrochloride.
Topotecan hydrochloride has the following structural formula:
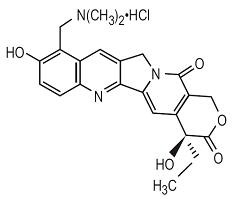
C23H23N3O5·HClM.W. 457.9
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
2
12.3 Pharmacokinetics
The pharmacokinetics of topotecan have been evaluated in cancer patients following doses of 0.5 to 1.5 mg/m2 administered as a 30-minute infusion.Topotecan exhibits multiexponential pharmacokinetics with a terminal half-life of 2 to 3 hours. Total exposure (AUC) is approximately dose-proportional.
Distribution
Binding of topotecan to plasma proteins is about 35%.
Metabolism
Topotecan undergoes a reversible pH dependent hydrolysis of its lactone moiety; it is the lactone form that is pharmacologically active. At pH ≤4, the lactone is exclusively present, whereas the ring-opened hydroxy-acid form predominates at physiologic pH. In vitro studies in human liver microsomes indicate topotecan is metabolized to an N-demethylated metabolite. The mean metabolite: parent AUC ratio was about 3% for total topotecan and topotecan lactone following IV administration.
Excretion
Renal clearance is an important determinant of topotecan elimination.
In a mass balance/excretion study in 4 patients with solid tumors, the overall recovery of total topotecan and its N-desmethyl metabolite in urine and feces over 9 days averaged 73.4 ± 2.3% of the administered IV dose. Mean values of 50.8 ± 2.9% as total topotecan and 3.1 ± 1% as N-desmethyl topotecan were excreted in the urine following IV administration. Fecal elimination of total topotecan accounted for 17.9 ± 3.6% while fecal elimination of N-desmethyl topotecan was 1.7 ± 0.6%. An O-glucuronidation metabolite of topotecan and N-desmethyl topotecan has been identified in the urine. These metabolites, topotecan-O-glucuronide and N-desmethyl topotecan-O-glucuronide, were less than 2% of the administered dose.
Effect of Gender
The overall mean topotecan plasma clearance in male patients was approximately 24% higher than that in female patients, largely reflecting difference in body size.
Effect of Age
Topotecan pharmacokinetics have not been specifically studied in an elderly population, but population pharmacokinetic analysis in female patients did not identify age as a significant factor. Decreased renal clearance, which is common in the elderly, is a more important determinant of topotecan clearance [see Dosage and Administration (2.3) and Use in Specific Populations (8.5)].
Effect of Race
The effect of race on topotecan pharmacokinetics has not been studied.
Effect of Renal Impairment
In patients with mild renal impairment (creatinine clearance of 40 to 60 mL/min.), topotecan plasma clearance was decreased to about 67% of the value in patients with normal renal function. In patients with moderate renal impairment (Clcr of 20 to 39 mL/min.), topotecan plasma clearance was reduced to about 34% of the value in control patients, with an increase in half-life. Mean half-life, estimated in 3 renally impaired patients, was about 5 hours. Dosage adjustment is recommended for these patients [see Dosage and Administration (2.3)].
Effect of Hepatic Impairment
Plasma clearance in patients with hepatic impairment (serum bilirubin levels between 1.7 and 15 mg/dL) was decreased to about 67% of the value in patients without hepatic impairment.Topotecan half-life increased slightly, from 2 hours to 2.5 hours, but these hepatically impaired patients tolerated the usual recommended topotecan dosage regimen.
Drug Interactions
Pharmacokinetic studies of the interaction of topotecan with concomitantly administered medications have not been formally investigated.
In vitro inhibition studies using marker substrates known to be metabolized by human P450 CYP1A2, CYP2A6, CYP2C8/9, CYP2C19, CYP2D6, CYP2E, CYP3A, or CYP4A or dihydropyrimidine dehydrogenase indicate that the activities of these enzymes were not altered by topotecan. Enzyme inhibition by topotecan has not been evaluated in vivo.
Cisplatin
No pharmacokinetic data are available following topotecan (0.75 mg/m2/day for 3 consecutive days) and cisplatin (50 mg/m2/day on day 1) in patients with cervical cancer.
[see Drug Interactions (7)]13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
Carcinogenicity testing of topotecan has not been performed. Topotecan is known to be genotoxic to mammalian cells and is a probable carcinogen. Topotecan was mutagenic to L5178Y mouse lymphoma cells and clastogenic to cultured human lymphocytes with and without metabolic activation. It was also clastogenic to mouse bone marrow. Topotecan did not cause mutations in bacterial cells.
Topotecan given to female rats prior to mating at a dose of 1.4 mg/m2 IV (about equal to the clinical dose on a mg/m2 basis) caused superovulation possibly related to inhibition of follicular atresia. This dose given to pregnant female rats also caused increased pre-implantation loss. Studies in dogs given 0.4 mg/m2 IV (about 1/4th the clinical dose on a mg/m2 basis) of topotecan daily for a month suggest that treatment may cause an increase in the incidence of multinucleated spermatogonial giant cells in the testes.Topotecan may impair fertility in women and men.
14 CLINICAL STUDIES
14.2 Small Cell Lung Cancer
Topotecan hydrochloride was studied in 426 patients with recurrent or progressive small cell lung cancer in 1 randomized, comparative study and in 3 single-arm studies.
Randomized Comparative Study
In a randomized, comparative, Phase 3 trial, 107 patients were treated with topotecan hydrochloride (1.5 mg/m2/day x 5 days starting on day 1 of a 21-day course) and 104 patients were treated with CAV (1,000 mg/m2 cyclophosphamide, 45 mg/m2 doxorubicin, 2 mg vincristine administered sequentially on day 1 of a 21-day course). All patients were considered sensitive to first-line chemotherapy (responders who then subsequently progressed ≥60 days after completion of first-line therapy). A total of 77% of patients treated with topotecan hydrochloride and 79% of patients treated with CAV received platinum/etoposide with or without other agents as first-line chemotherapy.
Response rates, response duration, time to progression, and survival are shown in Table 8.
Table 8. Efficacy of Topotecan Hydrochloride Versus CAV (cyclophosphamide-doxorubicin-vincristine) in Small Cell Lung Cancer Patients Sensitive to First-Line Chemotherapy
| Parameter | Topotecan Hydrochloride (n=107) |
CAV (n=104) |
|---|---|---|
|
aThe calculation for duration of response was based on the interval between first response and time to progression. |
||
| Complete response rate | 0% |
1% |
| Partial response rate | 24% |
17% |
| Overall respose rate | 24% |
18% |
| Difference in overall response rates | 6% |
|
| 95% Confidence interval of the difference | (-6 to 18%) |
|
| Response durationa (weeks) | n=26 |
n=19 |
| Median | 14.4 |
15.3 |
| 95% Confidence interval | 13.1 to 18 |
13.1 to 23.1 |
| hazard ratio (topotecan hydrochloride:CAV) (95% Cl) |
1.42 (0.73 to 2.76) |
|
| (P-value) | (0.3) |
|
| Time to progression (weeks) | ||
| Median | 13.3 |
12.3 |
| 95% Confidence interval | 11.4 to 16.4 |
11 to 14.1 |
| hazard ratio (topotecan hydrochloride:CAV) (95% Cl) |
0.92 (0.69 to 1.22) |
|
| (P-value) | (0.55) |
|
| Survival (weeks) | ||
| Median | 25 |
24.7 |
| 95% Confidence interval | 20.6 to 29.6 |
21.7 to 30.3 |
| hazard ratio (topotecan hydrochloride:CAV) (95% Cl) |
1.04 (0.78 to 1.39) |
|
| (P-value) | (0.8) |
|
The time to response was similar in both arms: topotecan hydrochloride median of 6 weeks (range 2.4 to 15.7) versus CAV median 6 weeks (range 5.1 to 18.1).
Changes on a disease-related symptom scale in patients who received topotecan hydrochloride or who received CAV are presented in Table 9. It should be noted that not all patients had all symptoms, nor did all patients respond to all questions. Each symptom was rated on a 4-category scale with an improvement defined as a change in 1 category from baseline sustained over 2 courses. Limitations in interpretation of the rating scale and responses preclude formal statistical analysis.
Table 9. Percentage of Patients with Symptom Improvementa: Topotecan Hydrochloride Versus CAV in Patients with Small Cell Lung Cancer
| Symptom | Topotecan Hydrochloride (n=107) |
CAV (n=104) |
||
|---|---|---|---|---|
| nb | (%) | nb | (%) | |
|
a Defined as improvement sustained over at least 2 courses compared to baseline. b Number of patients with baseline and at least 1 post-baseline assessment. |
||||
| Shortness of breath | 68 |
(28) |
61 |
(7) |
| Interference with daily activity | 67 |
(27) |
63 |
(11) |
| Fatigue | 70 |
(23) |
65 |
(9) |
| Hoarseness | 40 |
(33) |
38 |
(13) |
| Cough | 69 |
(25) |
61 |
(15) |
| Insomnia | 57 |
(33) |
53 |
(19) |
| Anorexia | 56 |
(32) |
57 |
(16) |
| Chest pain | 44 |
(25) |
41 |
(17) |
| Hemoptysis | 15 |
(27) |
12 |
(33) |
Single-Arm Studies
14.3 Cervical Cancer
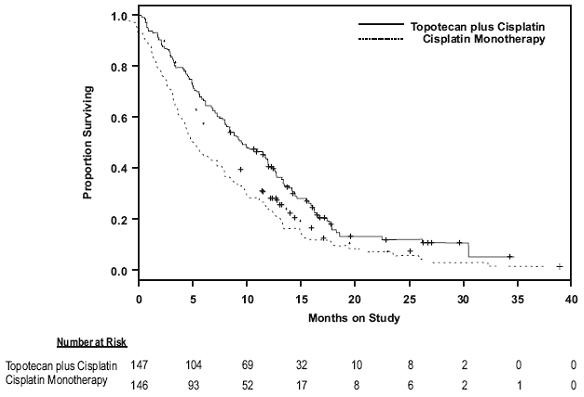
In a comparative trial, 147 eligible women were randomized to topotecan hydrochloride (0.75 mg/m2/day IV over 30 minutes x 3 consecutive days starting on day 1 of a 21-day course) plus cisplatin (50 mg/m2 on day 1) and 146 eligible women were randomized to cisplatin (50 mg/m2 IV on day 1 of a 21-day course). All patients had histologically confirmed Stage IV-B, recurrent, or persistent carcinoma of the cervix considered not amenable to curative treatment with surgery and/or radiation. Fifty-six percent (56%) of patients treated with topotecan hydrochloride plus cisplatin and 56% of patients treated with cisplatin had received prior cisplatin with or without other agents as first-line chemotherapy.
P
Figure 1. Overall Survival Curves Comparing Topotecan Hydrochloride Plus Cisplatin Versus Cisplatin Monotherapy in Cervical Cancer Patients
15 REFERENCES
- Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Health Care Settings. NIOSH Alert 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling Occupational Exposure to Hazardous Drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- American Society of Health-System Pharmacists. ASHP Guidelines on Handling Hazardous Drugs. Am J Health-Syst Pharm. 2006;63:1172-1193.
- Polovich M, White JM, Kelleher LO (eds.) 2005. Chemotherapy and Biotherapy Guidelines and Recommendations for Practice. (2nd ed) Pittsburgh, PA: Oncology Nursing Society.
16 HOW SUPPLIED/STORAGE AND HANDLING
Topotecan Hydrochloride for Injection is supplied as follows:
| Product No. |
NDC No. |
Strength | |
|---|---|---|---|
| 760210 |
63323-762-10 |
4 mg/vial |
Single-dose vial, packaged individually. |
| 760217 |
63323-762-17 |
4 mg/vial |
Single-dose vial, in packages of 5. |
Storage
Store the vials protected from light in the original cartons between 20° and 25°C (68° and 77°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
17.1 Bone Marrow Suppression
17.2 Pregnancy and Breastfeeding
17.3 Asthenia and Fatigue
Manufactured for:

Made in India
For Product Inquiry:
1-800-551-7176 or www.APPpharma.com
451215A/Issued: November 2010
PACKAGE LABEL.PRINCIPAL DISPLAY – Topotecan Hydrochloride for Injection 4 mg Single Dose Vial

TOPOTECAN
HYDROCHLORIDE
FOR INJECTION
For Intravenous Use
PACKAGE LABEL.PRINCIPAL DISPLAY - Topotecan Hydrochloride for Injection 4 mg Single Dose Vial , 5 pack

TOPOTECAN
HYDROCHLORIDE
FOR INJECTION
For Intravenous Use
Topotecan HydrochlorideTopotecan Hydrochloride INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||